- Clinical Trials

Volunteering
Volunteers are an integral part of the research process. People with a particular disease as well as healthy people both can play a role in contributing to medical advances. Without volunteers, clinical studies simply would not be possible.
People volunteer for clinical studies for many reasons. They may have a:
- Desire to improve medical care for future generations
- Connection to a certain disease or illness, whether through personal experience or through friends or family
- Personal interest in science
Participating is a choice
Volunteering for a clinical study is a personal choice. You have no obligation to do so, and participation is not right for everyone. After enrolling in a study, you may leave at any time for any reason.
Getting involved
- Participate in a clinical study at Mayo Clinic. By better understanding how to diagnose, treat, and prevent diseases or conditions, we help people live longer, healthier lives. Researchers need volunteers for a broad range of clinical studies. Find a clinical study .
- Connect with us. Eligibility requirements vary for each study and determine the criteria for participation. There is no guarantee that every individual who qualifies and wants to participate in a trial will be enrolled. Connect with the study staff directly as they are in the best position to answer questions and provide specific information regarding eligibility and possible participation. Contact information is found in each study listing.
- Join a national research volunteer registry. Health research changes peoples’ lives every day, but many studies end early because there are not enough volunteers. Researchers need both healthy people and those with all types of conditions. Funded by the National Institutes of Health, ResearchMatch is a first-of-its-kind registry that connects research volunteers with researchers across the country. Sign up at ResearchMatch.org .
Making an informed decision
- Informed consent. Before deciding to participate in a study, you will be asked to review an informational document called an informed consent form. This form will provide key facts about the study so that you can decide if participating is right for you. You must sign the informed consent form in order to participate in the study, though it is not a contract — you may still choose to leave the study at any time.
- Risks and benefits. All medical research involves some level of risk to participants. Risks and benefits vary depending on the particular study. To help you make an informed decision, the study team is required to tell you about all known risks, benefits and available alternative health care options.
- Ask questions. If you have questions when deciding to join a research study or at any time during it, ask a member of the study team. If your questions or concerns are not satisfactorily addressed, contact the study's principal investigator, the Mayo Clinic research subject advocate or the Mayo Clinic Institutional Review Board (IRB).
Protecting rights and safety
An independent group, the Mayo Clinic IRB , oversees all Mayo clinical studies that involve people, ensuring research is conducted safely and ethically. Members of the Mayo Clinic IRB include doctors, scientists, nurses and people from the local community.
In addition, Mayo Clinic has a research subject advocate who is independent of all clinical studies and is a resource for research participants. Contact the research subject advocate by email or at 507-266-9372 with questions, concerns and ideas for improving research practices.
Participation costs
Clinical studies may involve billable services and insurance coverage varies by provider.
Clinical studies questions
- Phone: 800-664-4542 (toll-free)
- Contact form
Cancer-related clinical studies questions
- Phone: 855-776-0015 (toll-free)
International patient clinical studies questions
- Phone: 507-284-8884
- Email: [email protected]
Clinical Studies in Depth
Learning all you can about clinical studies helps you prepare to participate.
Diversity in Clinical Trials
Mayo Clinic is keeping diversity and inclusion in focus for all clinical trials and addressing barriers to enrollment.
- Institutional Review Board
The Institutional Review Board protects the rights, privacy, and welfare of participants in research programs conducted by Mayo Clinic and its associated faculty, professional staff, and students.
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Postdoctoral Fellowships
- Training Grant Programs
- Publications
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.

An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

Healthy Volunteers
800-411-1222
Dial 711 to access telecommunications relay services.
Join us as a healthy volunteer! Your participation fuels clinical research and medical breakthroughs. Begin the registration process today to make a difference.
Begin Registration
The Vital Role of Healthy Volunteers in Medical Research
Healthy volunteers provide researchers with crucial data because their health information can be used as a comparison. In some studies, researchers need to compare healthy volunteers with people who have a specific disease or condition. Research with healthy volunteers is designed to develop new knowledge, not to provide direct benefit to study participants.
Healthy volunteers have always played a vital role in medical research. When developing a new technique such as a blood test or imaging device, we need clinical research volunteers to help us define the limits of "normal."
These volunteers are recruited to serve as controls for patient groups. They are often matched to patients on such characteristics as age, gender, or family relationship. They are then given the same test, procedure, or drug the patient group receives. Investigators learn about the disease process by comparing the patient group to the clinical research volunteers.
What's a "healthy volunteer" +
Someone with no known significant health problems who participates in research to test a new drug, device, or intervention is a "healthy volunteer" or "Clinical Research Volunteer".
Research participants include healthy volunteers and patient volunteers +
These volunteers are recruited to serve as controls for patient groups. They are often matched to patients on such characteristics as age, gender, or family relationship. They are then given the same test, procedure, or drug the patient group receives. Investigators learn about the disease process by comparing the patient group to the clinical research volunteers.
Why are healthy volunteers needed for clinical research? +
There are many reasons. When developing a new technique such as a blood test or imaging device, we need clinical research volunteers to help define the limits of "normal." Healthy volunteers are often matched to patients so that certain characteristics such as age, gender, or family relationship, are similar. Healthy volunteers are given the same test, procedure, or drug that the patient group receives. Investigators learn about the disease process by comparing the patient group to the clinical research volunteers.
How can I volunteer? +
One way to volunteer is to join the registry for the Clinical Research Volunteer Program. The program, created in 1995, is a resource that helps match potential research volunteers to clinical research studies at the NIH Clinical Center. To participate in the registry, we'll ask you to provide some basic information and give us permission to share that information with the research teams. If you are a potential match to a study's requirements, the study team will contact you.
How do I enroll myself or my child? +
You can contact us at 301-496-4763. Parents or guardians must call to register anyone under 18 years of age.
How do I find studies for healthy volunteers? +
To find studies for healthy volunteers go to http://clinicalstudies.info.nih.gov/ and search for studies using the word healthy . When you select individual studies, carefully review the study overview and eligibility requirements. If you meet the eligibility requirements, call 1-800-411-1222 (TTY 1-866-411-1010). We can provide participation details on up to three studies a day.
Ask about joining our Clinical Research Volunteer Program registry. To participate in the registry, we'll ask you to provide some basic information and give us permission to share that information with the research teams. If you are a potential match to a study's requirements, the study team will contact you.
Choosing to participate in a clinical trial is an important personal decision. For more information and answers to frequently asked questions about participating in clinical research, visit http://www.cc.nih.gov/participate.shtml . Compensation may be provided.
Provide suggestions to improve information about research participation.
Privacy Notice from the Office of Patient Recruitment

“The National Institutes of Health (NIH) offer excellent hospitality services for patients and participants in clinical studies. The nurses are kind, respectful, and always willing to listen to their patients' needs and make necessary accommodations. The patient rooms are equipped with comfortable amenities and are spacious enough to bring work supplies to make an extended stay productive. Overall, even during prolonged stays at the clinic, it is easy to adjust and create a routine tailored to the studies.”
Westminster, MD

Home » What to Expect From Our Volunteer Research Opportunities » Volunteer And Get Paid For Clinical Trials
Volunteer And Get Paid For Clinical Trials
Seize the chance to serve, while getting paid.
If you’re a student or a young individual who wants to earn extra income while finding purpose in service, becoming a volunteer of a paid clinical trial is a great way to achieve both objectives. Being a participant in a medical research study will surely add a sense of fulfillment and purpose to your life.
Biotrial offers a meaningful way to spend your time while earning money. Becoming a paid clinical trial volunteer is easy! Just register to be a participant for one of our online research studies and you can start getting paid during your first screening appointment.
By participating in our healthy studies, you offer hope for many people as you contribute to medical advancement. Our volunteers not only get involved in medical trials for money, they are also here for a purpose – an opportunity to help researchers find better treatments for diseases. In return, we provide compensation as a reward for your courage and contribution to the betterment of our medical state.
Why become a volunteer?
Get compensated for your time.
There are no out of pocket costs to participate in a study, instead, you get paid for doing them. All costs – from your travel expenses to your examination and medication, are covered by the sponsoring organization or individual. You will also get paid for studies as compensation for your time and service. The amount of payment you receive depends on several factors, including the type of trial, the length of your stay, and the number of times you visit the ambulatory. All participants will get paid for the studies they are involved in. Payment details, as well as other pertinent information, will be sent in an informed consent document.
Know that your safety comes first
Biotrial conducts Phase 1 and sometimes first-in-human studies with the highest standards of safety. Clinical trials have always been the cornerstone of medical research under the utmost condition that the safety of participants is ensured at all time. Since most of our clinical studies are in phase 1, we welcome healthy volunteers. A healthy volunteer is someone with no known illness and who is not taking any medication. It is their contribution that greatly impacts the future recipients for which these medicinal products and devices are intended.
Get the reward of helping others
At Biotrial, we consider our volunteers as modern research heroes. When you participate in a medical trial, you are doubly compensated. You get a monetary benefit, and you allow medical research to advance. In a few words, you take part in significant, life-saving causes. You might have a relative with a disease and want to help them. By joining Biotrial trials, you’ll gain a unique and empowering experience. Many of our volunteers report a sense of compassion and fulfillment after participating in our medical studies.
Learn about your health
Every year, hundreds of volunteers complete our onsite medical studies and get to know their medical condition much better. These individuals get frequent health check-ups and close monitoring of their body metabolism. During their stay, they have easy and frequent access to medical doctors and can ask all the questions they want. It is an opportunity to learn about a healthy diet and better understand medical diseases. Our clinical studies also offer the opportunity to better understand science and get more knowledge about diabetes, cancer, heart disease, and other illnesses that plague humanity.
We are looking for healthy volunteers who want to make a difference and get paid for research studies.
We need healthy men and women 18 to 80 all year round to become volunteers for our Phase I paid clinical trials. Our medical research studies compensate your time and effort. Register now to participate.

Sign up to become a clinical volunteer!
Complete the registration form and call us at 844-246-8459 . Our recruiters will be happy to help you, and it will take 5 minutes of your time to know if you are eligible. Your information will be kept strictly confidential.

Translational Research Support and Services for Scientists, Scholars, and Community Members
- Team Science
- Research Design & Analysis
- Informatics
- Clinical Studies & Trials
- Recruitment & Retention Support
- Community and Stakeholder Engagement
- Research Process Improvement
- T.5 Capacity in Medical Devices
- Biomedical and Health Data Sciences Collaborative (BHDSC)

Meet our staff
Education, training, and mentorship for new and seasoned researchers.
- Clinical and Translational Science Graduate Program
- T32 Fellowship Programs
- K Scholar Programs
- Junior Faculty Research Career Development Forum
- Professional Education
- Mentor Training
- Blue Star Investigator Certificate Program
- Clinical Research Staff Peer Mentoring Program
Dec 31, 10:00AM | Drop-In Sessions
Dec 24, 10:00am | drop-in sessions.
- Small Grants to Advance Translational Science (S-GATS)
- Open Opportunities
- Upcoming Opportunities
- Past Opportunities
- Career Development Awards
- Other Funding Sources
- Grant Writing Assistance
- Informatics Voucher Program
Since 2009, we’ve awarded 93 grants for innovative, interdisciplinary research through our Pilot Studies Program .
Tracking our progress towards acceleration, innovation and collaboration
- By The Numbers
- Success Stories
- Publications Referencing Tufts CTSI
- Beyond the Numbers

Featured Success Story
Building a culture and community of collaboration, what powers us, our partners.
- Commitment to Anti-Racism
- What is Translational Science?
- Broadly Engaged Team Science
- Annual Reports
- Academic Appointments
- How to Cite Tufts CTSI

Volunteer for Clinical Studies & Trials
Interested in participating in a clinical study or trial.
Research volunteers help doctors and scientists to test new drugs, therapies, medical devices and clinical and surgical methods. With your help, investigators can help to treat and cure medical conditions and diseases.
Whether you are a healthy patient, or someone looking to explore alternative treatments for an illness or condition, there is likely a clinical study or trial that needs your participation.
Before you sign up for a study, be sure to consult with your doctor. To learn more about participating in research, visit the Center for Information and Study on Clinical Research Participation (CISCRP) website .
Resources for potential study participants
The New England Research Subject Advocacy Group (NE RSA) published a series of brochures to support communication between researchers and participants. These resources provide useful information and helpful questions to think about and to ask before deciding to participate in a research study. Please visit Harvard Catalyst’s Research Subject Advocacy web page to download the brochures, available in 15 languages.
To contact the Research Participant Advocate , please call 617-627-4255.
Where to find a clinical study or trial
Tufts Medicine
Tufts University School of Dental Medicine
Cummings School of Veterinary Medicine at Tufts University
Jean Mayer USDA Human Nutrition Research Center on Aging
ResearchMatch
ClinicalTrials.gov
Research Participant’s Bill of Rights
As a participant in a clinical study or trial, you have the right:
- To be told why the study is being conducted.
- To be told who is funding the study.
- To be given an explanation of what will happen during the study, what is expected of you, and what will be different from non research medical treatment.
- To be given an explanation of any risks or discomforts that may be experienced from participating in the study.
- To be given an explanation of any benefits that may be expected from participating in the study.
- To be told, if treatment is part of the study, of other non-research treatment choices that are available and how they compare to participating in the study.
- To be given the opportunity to ask questions about the study or about participating in the study, before agreeing to participate and during the course of the study.
- To be told of your right to refuse to begin the study, or to change your mind and stop participating in the study after it has started. Your participation is completely voluntary. If treatment is part of the study, this decision will not affect your ability to receive non research treatment.
- To be told that you may refuse to answer any question.
- To have enough time to decide whether or not to participate and to make that decision without any pressure from the people who are doing the research.
- To be told, if treatment is part of the study, whether there are any costs to you associated with being in the study and whether you will receive any reimbursement for participating in the study.
- To be told who will have access to information collected about you, how the information will be used, and how the confidentiality of your information will be protected.
- To be told who to contact directly with questions about the research, about research related injury, and about your rights as a research participant.
- To be told, if the research is greater than minimal risk, whether any compensation and medical treatments are available should you have a research related injury, what the treatments are, and where further information may be obtained.
- To be told about new information learned during the study that might affect your safety or your willingness to continue to take part in the study.
- To receive a copy of the consent form if one is part of the study.
- Department of Health and Human Services
- National Institutes of Health

What is a healthy research volunteer?
A healthy research volunteer is a person with no known significant health problems who participates in a clinical research study to test a new drug, device, or intervention.
Research with healthy volunteers is designed to develop new knowledge, not to provide medical benefit to the healthy volunteer.
What is the purpose of the healthy volunteer registry?
The purpose of the healthy volunteer registry is to match potential research volunteers with current or upcoming clinical research studies at the NIH Clinical Center.
Joining the healthy volunteer registry is free of cost and you can cancel your registration at any time.
How can I join the healthy volunteer registry?
To join the healthy volunteer registry, you will be asked to provide basic information including:
- your contact details
- your characteristics and health
- permission for us to share your information with the clinical research study teams.
If you are a potential match for a study's requirements, the clinical research study team may contact you.
Will I receive compensation for participating in a clinical research study?
The NIH may compensate healthy research volunteers for their time and, in some instances, for the inconvenience of a procedure. You can inquire about the compensation rates when you are contacted by the clinical research study team.
Will the NIH report my compensation to the Internal Revenue Service?
The NIH reports compensation of $600 or more to the Internal Revenue Service and sends Form 1099-Other Income to healthy research volunteer at the end of the year.
If I have federal debts, can I still receive compensation?
Under U.S. law, compensation may be garnished by the U.S. Treasury if the research volunteer has outstanding debts to the federal government.
The NIH does not know if a research volunteer has an outstanding debt to the government and is not told when the U.S. Treasury garnishes compensation. The U.S. Treasury will notify the research volunteer directly in this instance.
After reading this information, you acknowledge and understand, NIH Privacy Notice and are willing to sign up for the registry?
By signing up, I agree to have my information included in a registry of individuals interested in being contacted about future research studies. I understand that I may be contacted in the future by phone or email about taking part in a study at the NIH Clinical Center. I understand that being included in the registry does not require that I participate in the research study.

Mailing Address
We are pleased that you are considering volunteering for clinical research. It is through clinical research that we advance medicine. Participation in a clinical research is completely voluntary.
Each study has specific requirements on who can participate, which may include age, gender, and medical diagnosis. Prior to enrolling in any study, a study team member will explain the study details to you.
Join The Contact Registry For Clinical Research
Sign up for the UCF College of Medicine’s Contact Registry for Clinical Trials to learn about clinical research and clinical trials in areas of interest to you. Whenever a study is opening in your interest area, we will let you know. You do not have to be a patient or have the disease, just need to be interested in learning more.
Information on Volunteering for Research from the United States Department of Health & Human Services, Office of Human Research Protections
- Becoming a Research Volunteer: It’s Your Decision — English (PDF)
- Becoming a Research Volunteer: It’s Your Decision — Spanish (PDF)
- PAB Recruitment Flyer — English (PDF)
Trials Open For Enrollment
Experienced patients enhancing research trials (expert) panel.
We are seeking patients in all disease areas to serve as subject matter experts/advisors for our research studies. The only qualification is your lived, personal experience of the disease. The commitment is less than 20 hours a year. To volunteer to be an EXPERT, reach out to [email protected] or 407 COM TRIALS.

Deciding Brains
Study: Deciding Brains
This study is looking at changes in memory for older adults.
Seeking: Persons ages 60-95 with memory issues but no dementia
PI: Dr. Nichole Lighthall, PhD; Dr. Mariana Dangiolo, MD

Contact Registry for Clinical Trials
Sign up for the UCF College of Medicine’s Contact Registry for Clinical Trials to learn about clinical research and clinical trials in areas of interest to you!
Study: Contact Registry for Clinical Trials
Seeking: Any adult
PI: Dr. Amoy Fraser, PhD

Diagnostic Test for Malignant Tumors
Study: Development of an Early Diagnostic Test for Malignant Tumors in Children with NF1
Seeking: Healthy persons to donate blood
PI: Dr. Cristina Fernandez-Valle

Cell-Free DNA CHIP for Diagnosing Cancer
Study: Cell-Free DNA CHIP for Diagnosing Cancer
Seeking: Healthy persons & persons with cancer to donate blood
PI: Dr. Kersten Schroeder
De-Identified Human Tissue Project
Study: De-Identified Human Tissue Project for Use with Translational, Biomedical Research at UCF College of Medicine and Burnett School of Biomedical Sciences
Seeking: Persons who will be doing a surgery to donate removed tissue
Translational Research Institute for Space Health (TRISH) Astronaut Studies
Limited participants- Astronauts Only.
Collaborating PI: Dr. Amoy Fraser, PhD
Extracellular Vesicles for Huntington’s Disease
Study: Extracellular vesicles as non-invasive biomarkers for Huntington Disease progression and huntingtin lowering therapy
Seeking: (1) Persons with HD; and (2) Healthy persons without HD to serve as controls
PI: Dr. Amber Southwell, PhD; Dr. Danish Bhatti, MD

Innate Immunity in Allergic Asthma
Study: Innate Immunity in Allergic Asthma
Seeking: Healthy persons (no asthma, no respiratory symptoms, no seasonal allergies) to donate blood
PI: Dr. Justine Tigno-Aranjuez, PhD

Pyoderma Gangrenosum
Study: A Randomized, Double-Blind, Placebo-Controlled, Multicenter, Adaptive, Phase III trial to Investigate Efficacy and Safety of Vilobelimab in the Treatment of Ulcerative Pyoderma Gangrenosum (IRX1001/IFX-1-P3-4)
This study seeks persons with ulcerative pyoderma gangrenosum to find out if a new investigational drug, vilobelimab is safe and effective in treating the condition.
Seeking: Persons diagnosed with Ulcerative Pyoderma Gangrenosum .
PI. Dr. Naveed Sami, MD

Baricitinib in Rheumatoid Arthritis (RA Branch)
Study: A Randomized, Controlled Pragmatic Phase 3b/4 Study of Baricitinib in Rheumatoid Arthritis
Seeking: Persons diagnosed in Rheumatoid Arthritis
PI: Dr. Shazia Beg, MD

FGF19 in Tumors
Study: Translational Utility of Tumor-Derived FGF19 in a Novel Blood-Based Endocrine Suppression Approach for Colorectal Cancer Screening
Seeking breast cancer and colorectal cancer patients.
PI Dr. Deborah Altomare, MD; Dr. Virgil Dawson, MD.
More Information

Microbiome in aging Gut and Brain (MiaGB)
Study: Microbiome in aging Gut and Brain (MiaGB) Study.
Seeking: Persons over 60 years old.
LSI Michal Masternak, PhD.
COVID-19 Chest Radiograph Imaging Repository for Artificial Intelligence Research
Study: Crowdsourcing an Open COVID-19 Chest Radiograph Imaging Repository for Artificial Intelligence Research.
Seeking: persons who had a chest Xray while diagnosed with COVID19.
PI: Dexter Hadley.

Blood Collection for COM and BSBS Scientists
Study: Blood Collection for COM and BSBS Scientists.
Seeking: adult blood donors .
PI Amoy Fraser, PhD.

Lake Nona Life Project
Study: Lake Nona Life Project.
Seeking: Persons who live, work, study, or play in Lake Nona.
Sign up at http://www.liveworkparticipate.com/
PI Eric Schrimshaw, PhD.

FINE REAL (Kerendia for CKD and T2D)
FINE REAL: A non-interventional study providing insights into the use of Finerenone in a routine clinical setting.
Seeking adults who are taking Kerendia (Finerenone).
PI Virgil Dawson, MD.
Trials Opening Soon For Enrollment
Pneumococcal carriage in the population.
Study: Post-pandemic genomic epidemiology of pneumococcal carriage among children and adults in the general US population
PI Dr. Taj Azarian, Co-I Dr. Virgil Dawson, Dr. Amoy Fraser
We are seeking HEALTHY adults over 50 years old and children under 5 years old to participate. You don’t need to be ill with pneumonia to participate. The 30-minute study visit involves a questionnaire and a nasopharyngeal swab. As thanks, we are giving a $30 Amazon gift card. To participate, contact [email protected] or 407 COM TRIALS.
Isolation of Human Cells
PI: Dr. Thomas Kean, PhD
Globospin Study
Study: A Prospective Observational Study of Patients receiving Dupixent for Prurigo Nodularis
Seeking: persons diagnosed with Prurigo Nodularis
PI: Dr. Naveed Sami, MD
Hair Analysis for Dialysis Patients
Study: Hair Analysis for Dialysis Patients. Open to dialysis patients
Seeking Veterans doing dialysis at the Orlando VA .
PI Ed Ross, MD.

Trials Closed For Enrollment
Study: A Biospecimen Samples Collection Study to Support the Development of the Detect STI Test
Seeking: *Limited Access* UCF Students Only
PI: Dr, Jane Gibson, PhD; Dr. Mary Schmidt-Owens, PhD

274S, 275C, 278C [DATA ANALYSIS ONLY]
*LIMITED ACCESS* Medical Students only
LIA-RESP-569
Study: Cobas® Liat SARS-CoV-2, Influenza A/B & RSV Nucleic Acid Test: Clinical Performance Evaluation
PI: Dr. Jane Gibson, PhD; Dr. Mary Schmidt-Owens, PhD

Targeting Immunometabolism to Control COVID-19
Study: Targeting Immunometabolism to control COVID19
PI: Dr. Hung Nguyen, PhD
Rheumatology Fellows AI Study
Study: Rheumatology Fellows’ Survey: Opinion on the Influence of Artificial Intelligence and Social Media
Seeking: Rheumatology students
PI: Dr. Neha Bhanusali, M.D.

Liquid Biopsy Collection Study
Collection of human blood for development of liquid biopsy assay
PI: Dr. Annette Khaled, PhD

Lupus Study
Study: A Phase 2 Randomized, Double-Blind, Placebo-Controlled Study to Evaluate Efficacy and Safety of Deucravacitinib (BMS-986165) in Participants With Active Discoid and/or Subacute Cutaneous Lupus Erythematosus (DLE/SCLE)
Seeking: Persons diagnosed with Active Discoid and/or Subacute Cutaneous Lupus Erythematosus (DLE/SCLE)
Depression Study.
Seeking: UCF students
PI: Dr. A’Naja Newsome, Ph.D
Study: A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study to Evaluate the Efficacy and Safety of Dupilumab in Adult Patients With Bullous Pemphigoid
Seeking adults with BP that is not adequately controlled
PI Naveed Sami
Sarcopenic Obesity in the Elderly
Study: Sarcopenic Obesity in the Elderly.
Seeking adults over age 60.
PIs Muthu Periasamy, PhD; Ali Rizvi, MD; and Mariana Dangiolo, MD.
The Eye in Orbit: UCF Adaptation
Study: The Eye in Orbit: UCF Adaptation.
Seeking: Selective enrollment. Not open to the public.
LSI Mehul Patel, MD.

ADDRESS, ADDRESS+
A Randomized, Double-Blinded, Placebo-Controlled Trial to Investigate the Efficacy, Safety, and Tolerability of Efgartigimod PH20 SC in Adult Patients with Pemphigus (Vulgaris or Foliaceus)
PI Naveed Sami, MD.

How to Volunteer for Clinical Trials: A Guide to Volunteering in Medical Research
Interested in volunteering for a clinical trial? Check out our detailed guide for clinical trial volunteers and those considering becoming one.
Reviewed by Giselle Leung , PharmD, BCGP
Published 20 December 2023
Clinical trials are a key research tool for advancing medical knowledge and patient care. They’re run to test various scientific developments – including medical interventions, surgical and radiological procedures, new devices, behavioral treatments, and preventive care.

However, a recent study by the Health Information National Trends Survey indicated that only 9% of Americans report having been invited to participate in clinical trials . And, of those who were invited, just 47% reported volunteering.
These low participation rates can result in trials failing – which is why more volunteers are so desperately needed.
Looking to participate in a clinical trial? Don't know where to start?
Our clinical trial platform can connect you with trials that match your needs and eligibility. Take the first step towards accessing cutting-edge treatments and start your search today to discover the potential benefits of participating in clinical trials.
Why are clinical trials important?
Researchers use clinical trials to determine what does and doesn’t work in humans – specifically what can’t be learned either in the laboratory or by assessing animals.
They can help develop medications and strategies for the treatment and prevention of disease, as well as ways to detect, diagnose, and reduce the chances of developing one.
Trials also help doctors decide whether the side effects of a new treatment are acceptable when weighed against the potential benefits.
How are studies approved for volunteer participation?
If a study involves an investigational drug, it must be approved by the U.S. Food and Drug Administration before volunteers are invited.
Studies are also screened for safety, ethics, and necessity by a team of physicians and scientists.
Why people choose to volunteer in clinical trials
People volunteer for many reasons, including personal interests, a desire to ‘give back’ and help others, or because they’re seeking support with a medical condition.
Access to new treatments
If an individual is suffering from a disease or illness, clinical trials may provide access to new, potentially life-saving treatments that are not yet available to the public. However, there is no guarantee that the trial will be successful.
Contributing to medical advancements
By participating in clinical trials, volunteers can improve researchers’ understanding of diseases and their treatment – potentially improving the care and lifespan of current and future patients.
Personal motivations for volunteering
Some people choose to participate in clinical trials due to an interest in science, a desire to help others, or because they feel a sense of empowerment over their own health.
Compensation
Clinical trials sometimes offer compensation for participation, which can be a motivating factor for certain individuals.
Support and care
If the volunteer has an illness or disease related to the trial, they can potentially meet people in a similar position, whilst being closely monitored by medical professionals. This can offer a sense of security and peace of mind.
Who can volunteer in a clinical trial?
Clinical trials aren’t limited to people suffering with an illness. In fact, both healthy people and those with an existing condition can participate, depending on the study’s requirements as detailed in the protocol.
Eligibility criteria for volunteering in clinical trials
Eligibility criteria vary, depending on the trial itself. However, there are some requirements common to all trials, such as certain health stipulations. Some trials might also require participants to meet a specific age, gender, or certain lifestyle requirements.
Volunteering in studies for healthy volunteers
Healthy volunteers are needed for early clinical research studies, helping to establish the safety, dosage, and side effects of new drugs or treatments.
Depending on the study, you might not be eligible if you use tobacco or illegal drugs, if you drink more than a certain amount of alcohol, or if you have a particular health condition. Pregnant and breastfeeding women may not be eligible to volunteer in some trials.
What to consider before applying to volunteer in a clinical trial
It’s important to understand the benefits and risks of a clinical trial before volunteering, as well as the costs, and commitments involved.
Is it safe to volunteer for clinical trials?
Clinical trials vary vastly in nature. And whilst some present a greater risk than others, it’s difficult to determine the safety of an individual study.
Before you volunteer, it’s wise to consider the severity of the illness or condition being studied, examine the study protocol , and investigate the qualifications of the research staff.
What are the benefits and risks of volunteering in a clinical trial?
By taking part in a clinical trial, you are advancing medical knowledge. You could help scientists find treatments for people suffering from chronic, serious, or life-threatening illnesses. Or indeed, for yourself. In some cases, you can benefit from receiving a thorough physical exam. And you might also receive compensation.
However, like routine medical procedures, clinical research trials do come with risks, some of which are more serious than others. The effectiveness and safety of the treatment being tested might be unknown, so it may not work as intended. In rare circumstances, this can result in participants requiring medical attention. Plus, there’s no guarantee that participating in a clinical trial will provide any benefits. However, these studies remain a vital part of the research process.
How much does it cost to volunteer in a clinical trial?
Some trials ask participants to pay for the cost of their own medical care, and to cover the cost of travel to and from the trial location, while others might provide it free of charge.
How much of your time will the clinical trial take?
Clinical trials can be time consuming, and you may need to make frequent visits to the study site. Each trial will vary, so find out as much as possible beforehand, and consider whether you have the time and flexibility to commit to the requirements.
Clinical trial volunteer rights and safety
Regulations and guidelines are in place to ensure that all clinical trials meet strict standards and that your rights and safety remain a priority.
All clinical trials must be approved and continuously monitored by an independent Institutional Review Board or a Human Rights Committee.
What protections exist for clinical trial volunteers?
Your individual rights include safe, considerate, and respectful care. This includes confidentiality, being given complete information about the protocols, risks, and benefits, as well as being assessed and treated if you suffer any pain or discomfort.
Informed consent
Before deciding to participate in a study, you will be asked to review an informed consent form. This form provides facts about the study, so you can make an educated decision about whether to participate.
If you want to proceed, you’ll need to sign the form, although this is not a contract and you can choose to leave the study at any time.
Safety monitoring
The trial must have measures in place to monitor participant safety , including regular check-ins and reporting any adverse events.
Confidentiality
All clinical trials are required to hold your personal and medical information in the strictest confidence. This includes compliance with patient privacy laws, such as HIPAA .
Right to withdraw
You have the right to withdraw from the trial at any time, and for any reason, without penalty.
Steps to take to become a clinical trial volunteer
There are several steps to take to locate and apply for suitable clinical trials.
Find a clinical trial
Many types of trials are available, so the first step is finding one that matches your interests, health status, and geographic location.
Review the eligibility criteria
You might need to be a certain age or gender, or meet specific health or lifestyle criteria. So always check the eligibility criteria carefully. Always consult with your doctor if any criteria are unclear.
Contact the clinical trial or study
After finding a clinical trial that interests you, either reach out to the study team or complete any required application forms to ensure your name is in the database of potential participants.
Pass the screening process
The clinical trial team will conduct an initial screening to assess your eligibility based on the study's inclusion and exclusion criteria.
This may include questions about your medical history, current medications, and other health-related factors. You might also be examined to assess your overall health.
Preparing to volunteer in a clinical trial
Volunteering in a clinical trial can be an incredibly rewarding experience. However, it’s important to be mentally and physically prepared.
Have realistic expectations
If you have a medical condition, remain realistic about what the trial can provide, as it may not offer a cure or solution. However, it is likely to provide valuable insights for the researchers involved.

Understand the study requirements
Understanding the clinical trial requirements is important as it can help alleviate any anxiety, as well as manage your expectations. These requirements might include the number of visits, tests, and procedures that are involved.
Talk to the study team
If you have any queries or concerns, the study team can answer your questions about the trial and provide more information.
What to expect during and after a clinical trial
Although all clinical trials are different, there are some common factors you can expect from most studies.
Day-to-day expectations
Most clinical trials include an exam of your health at the beginning, during, and end, which means you may receive more medical care than you would ordinarily. During the trial, you might also be asked to monitor yourself and report any adverse effects.
After the clinical trial ends
In general, at the end of a trial, the research team analyzes everyone’s results, and a summary is published in a report. You don’t usually receive your individual results.
If the trial shows promising results, it may continue onto the next phase, and you may be invited to participate further. This very much depends on the phase of the trial when you entered, and the feedback gained from it.
Where to find a clinical trial or study
Many hospitals and research centers conduct trials, so you could contact them or check their website to enquire about taking part.
Your doctor or healthcare provider may also be aware of trials that are looking for volunteers.
If you have a particular disease or condition, patient advocacy groups may have information about relevant clinical trials.
Several websites also specialize in connecting people with clinical trials. And some use social media and online communities to recruit participants, so it’s worth browsing online.
How can I find studies currently recruiting volunteers?
You can find information about research studies currently recruiting volunteers by viewing our clinical trials pages.
Our clinical trial services can help you find opportunities to participate in clinical trials as a healthy volunteer. You can contribute to the development of new treatments while also potentially receiving compensation for your time and effort. Sign up now and start making a difference.
Trials open for enrollment
If you're interested in participating in a clinical trial, we can help you find trials that are open for enrollment and match your needs and eligibility. By joining a clinical trial, you can potentially benefit from new treatments while also contributing to medical knowledge for future generations. Don't wait – start exploring your options today.
Trials opening soon for enrollment
Looking to be among the first to access new treatments through clinical trials? Our services can help you stay informed and up-to-date on trials opening soon for enrollment that match your needs and eligibility. Sign up now and be the first to know when these trials become available.
Clinical trials are vital for providing scientific insights that could potentially revolutionize patient care.
Even if they’re unsuccessful, the information gained by the study team can inform future research and trials, so every one of them is valuable.
By becoming a volunteer, you could play a role in improving the quality of life and prognosis for current and future generations. And potentially your own.
Share this article on social media:
Nitrosamine Impurities Affecting Drug-Drug Interaction Studies – Learn More

- Investigators
- Why Celerion?
- ESG at Celerion
- Management Team
- Global Locations
- News & Events
- Volunteer Recruitment
- Clinical Trial Design
- Feasibility
- Project Management
- Clinical Monitoring
- Medical Monitoring
- Immunogenicity
- Biosimilars
- Gene Therapy
- Metabolite Profiling
- Drug Development & Regulatory Affairs
- Modeling and Simulation
- Protocol Development
- Clinical Data Sciences
- Biostatistics
- Pharmacokinetics / Pharmacodynamics (PK/PD)
- Medical Writing and Reporting
- Respiratory Disease
- Metabolic Disease
- Renal / Hepatic Insufficiency
- Cardiovascular Safety Studies
- Tobacco Risk Evaluation
- Product Labeling Studies
- ECG Core Lab
- E-Lab Notebook
Fast, Faster, Fastest.
Rapid recruitment for healthy volunteers or specialty populations, experienced recruitment for early clinical development.
Celerion has an unbeatable record for study subject recruitment, both in healthy and patient populations. The goal is to find subjects and quickly get them screened and registered for your study. Our expert recruitment and call center teams are renowned in the industry for starting studies full and on-time. Each site (Belfast, Lincoln, and Phoenix) has a dedicated recruiting department. Our local community outreach coordinators assist the recruitment team with finding subjects for your study. Once found, our admissions and screening personnel work swiftly to get things started.
For studies requiring access to special populations, we enhance our proprietary database and internal efforts with a broad network of primary care physicians and hospitals to refer patients as appropriate. Celerion is ready to use our creative strategies to ensure on-time recruitment for your study.
Across our three clinical facilities, Celerion has customized and validated a database of over 130,000 healthy males and females with the ability to identify participants' demographic and medical profiles designed to match your study criteria. To augment our subject database and target a specific therapeutic area, Celerion applies a combination of mass media marketing and direct marketing outreach. These efforts also include accessing the large pool of subjects in our active recruiting database. Through our website and social media, we promote available studies and schedules, averaging over 15,000 hits a week.
Through a targeted mix of local media, including radio, print, television, health fairs, sponsorships and seminars, we will create a program to exceed your recruitment expectations.
On average, Celerion screens more than 2,300 subjects per month. This success rate is attributed to our experienced staff, our extensive recruiting efforts and our ability to understand both the study requirements and the local subject pool for each of our clinical facilities. Additionally, we use integrated recruiting teams across all sites, allowing for more synergistic recruiting efforts supporting multi-site studies.
Recruiting By the Numbers
We have the proof that we will exceed your recruitment expectations
Translating Your Science to Medicine.
Partner with us to get fast, actionable data you can rely on.
Related to "Volunteer Recruitment"
News & events.
11.05.24 By John Smith -->
02.11.2025 - 02.12.2025 By John Smith -->
02.03.2025 - 02.06.2025 By John Smith -->
Articles By John Smith -->
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih clinical research trials and you, why should i participate in a clinical trial.

Why Should I Join a Clinical Trial?
Have you considered participating in a clinical trial? Dr. Griffin Rodgers, director of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at the National Institutes of Health, discusses the role that clinical trial volunteers play in improving the health of current and future generations.
Clinical trials are part of clinical research and at the heart of all medical advances. Clinical trials look at new ways to prevent, detect, or treat disease.
Treatments might be new drugs or new combinations of drugs, new surgical procedures or devices, or new ways to use existing treatments.
The goal of clinical trials is to determine if a new test or treatment works and is safe. Clinical trials can also look at other aspects of care, such as improving the quality of life for people with chronic illnesses.
People participate in clinical trials for a variety of reasons. Healthy volunteers say they participate to help others and to contribute to moving science forward. Participants with an illness or disease also participate to help others, but also to possibly receive the newest treatment and to have the additional care and attention from the clinical trial staff.
Clinical trials offer hope for many people and an opportunity to help researchers find better treatments for others in the future.
- The basics about participating in clinical trials
- The impact of NIH research
This page last reviewed on May 30, 2019
Connect with Us
- More Social Media from NIH

- Volunteer Hub
- Packing List
- Refer a Friend
Volunteer Guide to taking part in a Clinical Trial

Volunteer Guide to Taking Part in a Clinical Trial at BioPharma Services
Clinical trials are an essential part of medical science research, a driving force for innovative advancements in preventing, detecting and treating disease and other health issues. Trials aim to determine if new drugs or combinations of drugs are effective and safe, discover different ways to utilize existing treatments and develop new medical devices or surgical procedures. They also examine other aspects of care, like enhancing quality of life for people with chronic illnesses and evaluating biopharmaceutical human abuse potential.
Medical science needs clinical research trials, and clinical research trials need volunteers. While many people understand that and may be interested in becoming a clinical trial volunteer, they may not be fully aware of the true benefits of clinical trials or lack knowledge of just what is involved in participation.
According to the Coalition for Clinical Trials Awareness, participation offers dual benefits for volunteers . The first is potential personal benefit. Participants may experience improved disease outcomes and could develop better health if they receive otherwise-unavailable medical therapies. The second is societal benefit. By moving a new therapy closer to market, clinical trial participation helps increase access for patients who will benefit from the new treatment option.
The United States’ National Institutes of Health describe similar benefits of clinical trial participation . These include helping others by contributing to knowledge about new drugs or procedures and gaining access to new research treatments before they are widely available, as well as receiving regular and careful medical attention from doctors and other health professionals during the study.
The opportunity to volunteer for a clinical trial is open to a wide range of people and populations. For an effective trial, researchers include participants from different age groups, ethnicities, health statuses and more. They need to see how different people respond to different treatments.
Healthy volunteers assist clinical trial researchers in developing new knowledge. Alternatively, patient volunteers have a known health problem and take part in research to better understand, diagnose or treat that disease or condition.
While clinical trial participation is important and rewarding , many people have questions or uncertainty about volunteering and may not realize what is involved. This guide is a resource of information about clinical trials, the process of participation and other things volunteers should know.
About Clinical Trials
Clinical trials are defined by the World Health Organization as “a type of research that studies new tests and treatments and evaluates their effects on human health outcomes.” They are used to test the safety and effectiveness of drugs and medical devices. Basically, every medicine or vaccine on the market once was the subject of a clinical trial.
There are numerous reasons clinical trials are conducted , from determining whether a new drug or device is safe and effective for people to learning how to safely use a treatment in a population for which it was not previously tested. Another purpose is to study different ways to use standard treatments or current, approved treatments so that they can become more effective, easier to use or decrease certain side effects.
In the United States and Canada, most clinical trials for drugs performed prior to the government approval process are randomized, double-blind and placebo-controlled , which is a combination known in medicine as the gold standard. There are various stages of clinical trials , and each one is led by a principal investigator , who is often a medical doctor, along with a research team comprising physicians, nurses, social workers and other health care professionals.
One type of clinical trial is a human abuse liability (HAL) study . Also referred to as Human Abuse Potential (HAP) studies, they are designed to evaluate whether a new drug product has abuse potential, including among individuals with a history of recreational use of drugs of abuse. These Phase 1 clinical trials studies consist of pharmacology assessments , which provide unique information relevant to central nervous system-active drugs and are an important component of product labeling and scheduling recommendations of new drugs.
Concerns About COVID-19
If you’re interested in volunteering to participate in a clinical trial but are wary of doing so during the coronavirus pandemic, you’re not alone. Nearly 1 in 5 U.S. patients with cancer reported that they were less likely to enroll in a clinical trial due to fears of COVID-19 exposure.
However, regulatory agencies in both Canada and the U.S. have proactively taken steps to ensure that patients participating in clinical trials are exposed to as little risk as possible amid the pandemic. The National Cancer Institute and the U.S. Food and Drug Administration (FDA) are allowing for remote consent and virtual (telehealth) visits, while Health Canada has developed a new regulatory pathway through an interim order for clinical trials on medical devices and drugs relating to COVID-19 .
Steps for a Clinical Trial Volunteer
The first step to participating in a clinical trial is finding one that matches your interests, health status and geographic location. On ClinicalTrials.gov, there are currently 367,057 registered research studies in all 50 U.S. states and in 219 countries, including more than 4,500 for COVID-19 . Health Canada’s Clinical Trials Database is available to assist Canadians in finding clinical trials, which are required to follow Division 5 of the Food and Drugs Regulations and good clinical practices .
Once you’ve come across a trial in which you’d like to participate, you can contact the trial or study coordinator or fill out any necessary forms to add your name to a database of potential enrollees. Simply follow the instructions for that particular clinical trial.
Next, review eligibility criteria to find out if you qualify for that particular study. Your eligibility may be based on your age, gender, overall health, type and stage of a disease, treatment history and other conditions. It’s also important to understand the protocol for the clinical trial , including: ● The types of volunteers who may enter the study ● The schedules of tests and procedures ● The drugs involved ● The dosages or amount of the drug ● The length of the study ● What the researchers hope to learn from the study
Protocol requirements for clinical trials are designed to ensure participants are treated as safely as possible. Therefore, volunteers must agree to these terms to participate.
Another important step to enrolling in a clinical trial is speaking with your physician or other health care provider to discuss its benefits and risks. He or she can give you information on what to expect from the clinical trial process and provide guidance on whether a specific study is a good option based on your health status and current form of care. As with any treatment, be sure to keep your health care provider apprised of your participation in a clinical trial.
Although the U.S. government requires researchers to give prospective participants complete and accurate information about what will happen during the study, you might want to discuss any questions or concerns you have about it with the trial coordinator. Specific questions could include: ● What is being studied? ● How much time is required to participate? ● Is there compensation for travel and other expenses? ● How often will I have to visit the study site? ● What risks or side effects might I experience? ● What tests and procedures are involved? ● Will hospitalization be required? ● What are the chances I receive the experimental treatment or the placebo ? ● Will the results of the study be provided to me? ● How will you ensure my privacy?
Summary of the Process
If you qualify for a specific clinical trial, the first step of the process will be pre-screening. You’ll be asked via telephone, an online questionnaire or in-person about your medical history and overall health. The content used by the study staff has to be pre-approved by the Institutional Review Board (IRB) , an appropriately constituted group that has been formally designated to review and monitor biomedical research involving human subjects.
Next, you’ll be asked to provide informed consent, a process during which you agree to enroll in the trial and share your health data with its researchers. You’ll be given an information sheet with important information about the trial, including an explanation of the purposes of the research, the expected length of time for participation, description of any predictable risks, resources for additional information and more.
After completion of informed consent comes the screening process, which is the utilization of tests to verify you meet the criteria to participate in the trial and are eligible to be enrolled in it. These may include laboratory, diagnostic and/or cognitive tests, along with a physician examination. Oftentimes, once this process is completed, you’ll meet with at least a portion of the trial staff, including the clinical coordinator, who can address any more questions you have about the study.
Once you’re enrolled, you’ll be asked to follow clinical trial protocol. If you’re screened to determine your eligibility for the trial but don’t participate, you’re not considered enrolled . For enrolled participants, researchers employ randomization , which is the process of assigning trial subjects to investigational treatment or control groups using an element of chance to reduce bias in determination.
The number of study visits varies for each clinical trial, and they can occur in a variety of settings , including inpatient units, outpatient clinics, lab draw locations, diagnostic testing locations, research labs and Clinical Research Centers (CRCs). The clinical trial coordinator should inform you ahead of time about the time and date of your baseline visit, how long each visit will take, what will occur during each one, any specific instructions to follow before and after the visit and more.
Learning About Study Results
Once the trial is completed, you likely won’t have access to the drug or device being tested, depending on when or if it receives regulatory approval. Researchers first have to carefully examine information collected during the study before making decisions about the meaning of the findings and whether or not further testing is needed.
Often, there’s a follow-up period after the trial, during which the study team monitors an individual’s health over time. If a potentially dangerous effect or issue is found in any participants after treatment, researchers must report that information to you as quickly as possible.
Some researchers will notify you upon request about the trial’s results, but they are not required to do so. However, results usually are published in peer-reviewed scientific journals. You can also find published study results by searching for the study’s official name or Protocol ID number in the National Library of Medicine’s PubMed database and the National Institutes of Health registry.
BioPharma Services is a Contract Research Organization that conducts research studies across all medical disciplines. We have offices and trial sites in Toronto, Ontario and St. Louis, Missouri. If you’re interested in learning more about clinical trials or becoming a volunteer, please visit our Volunteer Hub to see our current research studies.
Popular Posts
Clinical Trials are divided into 4 phases. Phase 1 and 2 trials constitute early phase trials, Phase 3 and 4 research studies are late-phase trials.
The primary objective of Phase 1 studies is to determine the correct drug dosage by evaluating drug safety and determining if there are any side effects. Phase 1 trials are conducted in healthy volunteers.
Phase 2 studies also study the safety of a drug but focus on evaluating its effectiveness. These studies can be conducted in healthy volunteers or in individuals who have a certain disease or condition.
A Clinical trial is a process which is performed to determine whether an investigational drug, device or therapy is safe and effective. In early phase research (i.e. Phases 1 and 2), the safety and effectiveness of the drug will be evaluated in healthy volunteers.
An investigational drug can also be called an experimental drug and is being studied to see if your disease or medical condition improves while taking it. Scientists are trying to prove in clinical trials:
- If the drug is safe and effective.
- How the drug might be used in that disease.
- How much of the drug is needed.
- Information about the potential benefits and risks of taking the drug.
In order to evaluate the drug profile, we need to understand its pharmacokinetics. This is essentially how the body reacts to a drug after its administration through the mechanisms of absorption, distribution, as well as the metabolic changes. Therefore, blood draws are collected at various time points to better understand this mechanism. Each study requires a specific number of blood draws and total blood volume. These values will be provided to you and clearly stipulated in the informed consent form (ICF). The amount of blood that will be taken is outlined in the ICF.
As every study is testing an investigational product, there may be side effects. You will be provided with a list of side effects that have been reported in previous trials (if any), so you can make an informed decision whether or not to participate in the trial. During the trial you will be required to immediately inform clinic study staff of any adverse effects that you are experiencing. These side effects usually resolve upon discontinuation of the study drug.
Volunteers are compensated and the amount varies depending on the length of the clinical trial, length of stay and number of follow-up visits. The compensation is not specifically related to the risks or type of drug involved in the trials or studies. Every study is different and therefore, the compensation will vary. Study volunteers may receive between $1000 to $4000 for a trial (based on the factors listed above).
Food – Clinical trials are conducted in a controlled setting which means that all food is provided and trial volunteers receive standardised meals. Individual meal plans or meal preferences cannot be provided. If you have any food allergies or hypersensitivity to food product(s) that are clinically significant or life-threatening you may not be able to participate in a trial. Please contact us to discuss any food issues.
Accommodation – During your in-clinic stay you will share sleeping areas with other volunteers who are of the same gender. Similar to a hospital setting, supervision will be provided to ensure that your health and safety are being monitored.
Details of the duration of a study can be found on the Volunteer Hub .
Before you decide whether or not to take part in a clinical research study, you will be required to read and understand the information provided in an Informed Consent Form (ICF). The ICF describes the clinical research study and the nature of the investigational product to be used, including:
- Your rights and responsibilities as a study participant.
- What you will be asked to do during study participation.
- The potential risks that you should be aware of.
During this process, you will have the opportunity to discuss and ask questions related to the conduct of the clinical research study with the study doctor/ study staff. You are under no obligation to participate and your decision to take part in a clinical trial is voluntary.
Yes, we encourage you to bring items that will help to pass the time while you are in the clinic. You can use the time to study or work or catch up on the movies you’ve been meaning to watch.
Wondering what to pack before your site visit? Visit our Packing List page to learn more.
You will receive compensation as outlined during the Informed Consent process. Typically, you will receive compensation once all study visits have been completed. If, for any reason, you do not complete all study visits, your compensation will be on a pro-rated basis (i.e. for the time that you have participated in the trial)
We provide a clean, safe socially distance sleeping environment, in a dormitory of hotel room style. Watch video
All aspects of clinical trials are closely monitored including the food and drink consumed by participants. While you are in the clinic, you will be provided with all meals as per the study guidelines. You will need to eat all the food provided to ensure the guidelines are met. Watch video
We respect and value the privacy of our volunteers. View our Privacy and Cookie Policy here.
Privacy Preference Center
You can unsubscribe at any time. For more details, please read our Privacy Policy.
Add Text and Images to Your Form With Ease
To get started, replace this text with your own.
Thank you for your interest in becoming a Research Participant! To get started, please complete the Volunteer Registration Form.
Please do NOT fill out this form or 'Request a Follow-up' if you are not a business-related inquiry.
Hey there! Interested in working at BioPharma Services? Please visit our Careers Page to browse our current openings or, email your inquiries to [email protected]
Please do NOT fill out this form if you are not a business-related inquiry.

- Diversity in Clinical Research
- Clinical Research for Older Populations
- Clinical Research for Compensation
- Clinical Trial Information for Parents
- What Is A Clinical Trial?
Idaho Paid Clinical Trials
Match to clinical trials, popular cities, search clinical trials in idaho by condition.
This content is for informational and educational purposes only. It is not intended to provide medical advice or to take the place of such advice or treatment from a personal physician. All readers/viewers of this content are advised to consult their doctors or qualified health professionals regarding specific health questions. Policylab.us does not take responsibility for possible health consequences of any person or persons reading or following the information in this educational content. All viewers of this content, especially those taking prescription or over-the-counter medications, should consult their physicians before beginning any nutrition, supplement or lifestyle program
How to Participate in Cancer Research
Everyone can support cancer research by joining a study, donating tissue, and volunteering.
Prevention trials need healthy people and those who no longer have cancer.
Healthy volunteers can help to detect cancer early in screening trials.
Most cancer clinical trials help to find new treatments for cancer patients.
People with and without cancer can join these non-treatment studies.
More Ways to Get Involved
Help find new ways to reduce or prevent symptoms of cancer.

Researchers can use your existing medical records and specimens to advance cancer research.

Donate to cancer by volunteering, giving money, donating tissue, or being in a research study.
Season 2 of the Buck podcast is live! Learn more.

by Buck Institute
November 11, 2024 . BLOG
Excited about participating in our clinical trials?
Here’s what you need to know about our clinical research core, part 2.
In our last blog we talked about the mission of the Buck’s Clinical Research Core (CRC) and how it links to our basic research. Here, in Part 2, we discuss how the CRC came about and how it is growing.
The Clinical Research Core (CRC) all began with a fascination with ketone bodies. The co-heads of the CRC, Director of Translational Science Brianna Stubbs and Assistant Professor Newman, MD, PhD have studied ketones throughout their careers. Stubbs did her PhD studies on ketone drinks in humans, and Newman has done ketone research in animals for a decade. Studies have shown that ketone body supplements may provide a number of health benefits, without having to go on the traditional strict ketogenic diet. But whether this will pan out in clinical trials, along with the important details of which ketones, how much, and how different people respond, isn’t known.

The CRC launched in 2022 with a single study on a ketone drink that was commercially available. That study, dubbed BIKE (Buck Institute Ketone Ester), determined the safety of daily consumption of ketone ester beverages in older adults . The overwhelmingly enthusiastic response of participant volunteers helped it finish in under a year, from first to final study visit. It has already resulted in two accepted publications, with two more progressing through peer review. A wide array of aging biomarkers and multi-omic analyses are also underway, work that will bear scientific fruit for years to come.
At around the same time, the Buck began a clinical trial of ketones for use in the military, called STAK (Strategies to Augment Ketosis), in collaboration with Ohio State University. The portion of STAK being run at Buck tests how individuals of various ages with differing health conditions respond to a ketone ester . “Those two studies, STAK and BIKE, are the core that started us off because of our shared scientific and practical experience studying ketone drinks,” says Stubbs.
Federal funding is accelerating the program
After BIKE was established and running, $3.5 million in federal funding was obtained to launch the much larger TAKEOFF (Targeting Aging with Ketone Ester in Older Adults for Function in Frailty) trial, testing whether taking ketone drinks can help with physical function in people with early signs of frailty, a medical condition of reduced function and health in older people. The Buck is leading the study, which also involves Ohio State University and the University of Connecticut as collaborating enrollment sites, and the San Francisco Coordinating Center which makes sure things run smoothly between sites.
“TAKEOFF is also helping to launch our efforts to diversify geroscience clinical trial participation. Geroscience is a unique field that affects every one of us as we age, and it’s important to us that everyone in our community can be involved,” says Newman. TAKEOFF will enroll Spanish-speaking participants, and the CRC team includes several bilingual study staff. With TAKEOFF soon getting off the ground, the Buck CRC has begun an outreach program to local community groups serving older adults, especially in Latino communities.
In addition to the ketone drink studies, the number of trials keeps growing and moving in different directions. Current trials include an exploration of the effects of exercise on aging to understand better how exercise improves health and increases lifespan, and using a supplement to reduce the damaging effects of sugar metabolism to determine if it can improve hormonal health in postmenopausal women.
Meet the team and find out what they love about their jobs
None of this could be accomplished without the team that has been assembled, which includes senior nurses with decades-long experience in dealing with vulnerable older patients in medical environments, physicians experienced in both clinical research and the care of older adults, and clinical research associates with many years of clinical, laboratory and research experience to carry out the day-to-day activities. The team collects samples, including biological specimens, physical measurements, and questionnaires testing cognitive levels. They also process the samples in the laboratory, create computerized databases of the collected results and analyze the various data using self-created computer programming.
In addition to Stubbs and Newman, and new part-time physicians, the current CRC team includes a small but mighty team. Read on to hear what they have to say about their work at the Buck

Laura Alexander, RN, [BSN, CFCS], Clinical Research Nurse
“In my most recent position as Director of Nursing for a Long-term Care Facility I was shocked and frustrated at the lack of care that my patients received regarding their feet. I decided to change that by becoming a Certified Foot Care Specialist, and I am working at the Buck Institute for Aging to hopefully continue to contribute to the improvement of the care of the aging population.”

Thelma Y. García, Ph.D., Administrative Director of CRC
“I am excited to interact with a group of amazing people that are truly passionate about the work we do. I hope to continue to apply my expertise in research project management and a commitment to diversity, equity, inclusion, and belonging to excel in cultivating a collaborative culture that propels forward the frontiers of aging research.”

Ester Hernandez, Bilingual Clinical Research Associate
“My goal is to make vital information more accessible to the Latino community, ensuring they fully understand the trial processes and outcomes. Additionally, I aim to educate the community by bridging language barriers, which will ultimately help increase their participation and representation in clinical research.”

“I got involved in working on clinical trials when I was a 19-year-old undergraduate. That experience ignited a passion for clinical research. Losing a close relative to Alzheimer’s sparked a deep personal interest in neurological disorders and how they impact individuals and families. I’m bilingual and am excited to do outreach to a community that is often left out of the research equation.”

“It's always amazing to realize how multidisciplinary, talented and competent the staff are to make sure all aspects of the trials are completed. Here at the Buck we have created from the ground up a clinical unit that can do any aspect of the trial, from volunteer interactions to laboratory processing of the clinical samples, without a hitch.”

“I like the fact that we really work well together as a team, and I enjoy contributing my intravenous and nursing skills to these studies. Our Clinical Research Team appreciates the volunteers very much - our learning about various aspects of aging depends on them. I am an older nurse with 38 years of experience, so these studies are important to me as I age further.”

“I am very grateful to have grandparents who are in their 70s who have aged very well; my grandfather helped me carry a piano up to my apartment at age 75 and I want more people to be able to live like that into their older age. Some of the trials we are doing will help us learn more about the aging body and learn more about how we can augment it, or what we can do to benefit people as they age and increase health span.”
For more information on our clinical trials and to show your interest in participating, please go to: https://www.buckinstitute.org/clinical-trials/
Like our blog?
Get notified whenever there's a new post!
- Comments This field is for validation purposes and should be left unchanged.
Science is showing that while chronological aging is inevitable, biological aging is malleable. There's a part of it that you can fight, and we are getting closer and closer to winning that fight.
Support the buck.
We rely on donations to support the science that we believe will add years to people's lifespan and decades to their healthspan.
Keep up with our research. Sign up for our newsletter.
Atlant Clinical is a strong multinational Contract Research Organization (CRO) offering a full range of clinical trial and relevant support services throughout the US, Europe, Russia, and Middle Asia. We are a highly professional team with extensive clinical experience (Phases I–IV) in over 15 therapeutic areas.
Our mission is to aid pharmaceutical and biotech companies with the delivery of new drugs and medical products to patients in the shortest time possible. We take pride in the utmost quality of service, transparency of operations, and full commitment to improving the lives of people around the world.
WIDE GEOGRAPHICAL COVERAGE
On the ground presence around the world
OPERATIONAL FLEXIBILITY
We’re always ready to tailor services directly to your needs
Impeccable service and quality across all of our operations
STUDY RESCUE
We’re prepared to step in if you need us – patient recruitment within tight timelines and unique study parameters
RISK-SHARING
We’re not just a CRO – We’re your partner
We are always glad to meet you at international and local exhibitions, conferences and forums dedicated to clinical trials.
OUR SERVICES
Established in 2007, our international CRO offers extensive experience and expertise in Phase I-IV clinical trials and a vast number of support services.
ATLANT CLINICAL AT A GLANCE
Atlant Clinical, a reliable and efficient provider of clinical research services in the USA, Europe, Meddle Asia and Baltic States.
We conduct trials ICH GCP guidelines, all applicable international and local regulations and legislation, and any requirements specific to the project.
WHY ATLANT CLINICAL
With competition fierce and budgets tight, the world's leading pharmaceutical and biotechnology companies need a clinical trials partner they can rely on.
Latest Events
Bio 2019,philadelphia, pa., 3rd life sciences and pharmaceuticals symposium, new york., adam smith conference, clinical trials in russia, moscow., bringing drugs to the russian and the eurasian economic union markets, moscow, russia., scope 2019, orlando, achondroplasia parents will arrange the round table, moscow, russia., dia 2019, san diego, ca., view all events.
Testing the Combination of New Anti-cancer Drug Peposertib with Avelumab and Radiation Therapy for Advanced/Metastatic Solid Tumors and Hepatobiliary Malignancies
- Study HIC# : 2000038343
- Last Updated : 11/12/2024
This phase I/II trial studies the best dose and side effects of peposertib and to see how well it works with avelumab and hypofractionated radiation therapy in treating patients with solid tumors and hepatobiliary malignancies that have spread to other places in the body (advanced/metastatic). Peposertib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Immunotherapy with monoclonal antibodies, such as avelumab, may help the body’s immune system attack the cancer, and may interfere with the ability of tumor cells to grow and spread. Hypofractionated radiation therapy delivers higher doses of radiation therapy over a shorter period of time and may kill more tumor cells and have fewer side effects. Giving peposertib in combination with avelumab and hypofractionated radiation therapy may work better than other standard chemotherapy, hormonal, targeted, or immunotherapy medicines available in treating patients with solid tumors and hepatobiliary malignancies.
For more information about this study, including how to volunteer, contact:
Katie Wildman
- Phone Number: 1-203-615-2783
- [email protected]
Help Us Discover!
You can help our team find trials you might be eligible for by creating a volunteer profile in MyChart. To get started, create a volunteer profile , or contact [email protected] , or call +18779788343 for more information.
Eligibility Criteria
Inclusion criteria.
- PHASE 1: Patients must have a histologically confirmed metastatic or locally advanced unresectable solid tumor that has progressed on or after available standard of care therapy or for which no acceptable standard of care therapy exists, or in which the patient declines standard of care therapy (each patient that declines standard of care therapy will be documented in the case report form)
- PHASE 2: Patients must have a histologically confirmed metastatic or locally advanced unresectable cholangiocarcinoma/gallbladder carcinoma that has progressed on at least 1 prior standard of care therapy or for which no acceptable standard of care therapy exists, or in which the patient declines standard of care therapy (each patient that declines standard of care therapy will be documented in the case report form)
- Age >= 18 years * Because no dosing or adverse event data are currently available on the use of M3814 in combination with avelumab in patients < 18 years of age
- Eastern Cooperative Oncology Group (ECOG) performance status =< 2 (Karnofsky >= 60%)
- Patients with at least 1 index lesion to irradiate for whom palliative radiation treatment is indicated (including but not limited to pain and/or symptom control, prevention of disease -related complications, and preservation of organ function). Lung and liver lesions are preferred, though alternate lesions may be considered after discussion with trial principal investigator (PI). Up to 2 lesions may be considered for irradiation provided at least 1 lesion will receive the study treatment of total of 60 Gy and all prescribed irradiation will be completed within the radiation window
- Patients with at least 1 Response Evaluation Criteria in Solid Tumors (RECIST) measurable lesion (to be unirradiated) (defined as those accurately measured in at least one dimension, with the longest diameter to be recorded for non-nodal lesions and the shortest diameter for nodal lesions). Measurable is defined as at least 10 mm in longest diameter for solid tumors, at least 15 mm in shortest diameter for lymph nodes
- Patients must be willing to undergo fresh biopsies at baseline (as opposed to using archival tissue), in the event their baseline tissue was obtained > 12 months prior to study consent and/or they are randomized to the gamma H2AX pNBS1 multiplex IFA assay
- Absolute neutrophil count (ANC) >= 1,500/mcL
- Platelet count >= 100,000/mcL
- Hemoglobin >= 9.0 g/dL
- Serum creatinine =< 1.5 x upper limit of normal (ULN) OR calculated serum creatinine clearance (glomerular filtration rate [GFR] can be used in place of creatinine or creatinine clearance) >= 60 mL/min for participants with creatinine levels > 1.5 x institutional ULN * Calculate serum creatinine clearance using the standard Cockcroft-Gault formula
- Serum total bilirubin =< 1.5 x ULN or direct bilirubin =< ULN for participants with total bilirubin > 1.5 x ULN * Patients with known Gilbert disease with serum bilirubin level =< 3 x ULN are eligible
- Aspartate aminotransferase (AST) (serum glutamic-oxaloacetic transaminase [SGOT]) and alanine aminotransferase (ALT) (serum glutamate pyruvate transaminase [SGPT]) =< 2.5 x ULN or =< 5.0 x ULN for patients with hepatobiliary tumors/liver metastases
- Albumin >= 2.8 g/L
- International normalized ratio (INR) or prothrombin time (PT) or activated partial thromboplastin time (aPTT) =< 1.5 x ULN * This applies only to patients not receiving therapeutic anticoagulation; patients receiving therapeutic anticoagulation should be on a stable dose
- Participants must have the ability to swallow and retain oral medication and not have any clinically significant gastrointestinal abnormalities that might alter absorption
- Female patients of childbearing potential must have a negative urine or serum pregnancy test within 72 hours prior to receiving the first dose of study medication. If the urine test is positive or cannot be confirmed as negative, a serum pregnancy test will be required. The effects of M3814 and avelumab on the developing human fetus are unknown and there is the potential for teratogenic or abortifacient effects. For this reason, women and men of child-bearing potential must agree to use adequate contraception (hormonal or barrier method of birth control; abstinence) prior to study entry, for the duration of study treatment, and for 6 months after completion of M3814 and avelumab administration. Should a woman become pregnant or suspect she is pregnant while she or her partner is participating in this study, she should inform her treating physician immediately. Because there is an unknown but potential risk for adverse events in nursing infants secondary to treatment of the mother with M3814 and avelumab, breastfeeding should be discontinued if the mother is treated with M3814 and avelumab
- Ability to understand and the willingness to sign a written informed consent document. Participants with impaired decision-making capacity (IDMC) who have a close caregiver or legally authorized representative (LAR) and/or family member available will also be eligible
Exclusion Criteria
- PHASE I: Patients who have received prior anti-CTLA-4, anti-PD-1, anti-PD-L1 or other immune checkpoint inhibitor therapeutic antibodies or pathway-targeting agents
- PHASE II: Patients who have received prior anti-CTLA-4, anti-PD-1, anti-PD-L1 or other immune checkpoint inhibitor therapeutic antibodies or pathway-targeting agents with the following exceptions: * Patients who have only received previous durvalumab (anti-PD-L1) in combination with gemcitabine +/- cisplatin as part of first line therapy (TOPAZ-1 regimen) are eligible * Patients who have only received previous pembrolizumab (anti-PD-1) in combination with gemcitabine +/- cisplatin as part of first line therapy (KEYNOTE-966 regimen) are eligible
- Patients who have had chemotherapy, definitive radiation, biological cancer therapy, or investigational agent/device within 21 days of first planned dose of study therapy (within 14 days for palliative radiation). Previously irradiated lesions may be re-irradiated provided there is disease progression in the irradiated lesion and the prescribed radiation dosage can safely be re- administered
- Patients who have not recovered from adverse events due to prior anti-cancer therapy (i.e., have residual toxicities > Common Terminology Criteria for Adverse Events [CTCAE] grade 1) with the exception of alopecia
- Patients with untreated/uncontrolled central nervous system (CNS)/leptomeningeal disease. Patients with asymptomatic, treated CNS disease are eligible if the treating physician determines that immediate CNS specific treatment is not required and is unlikely to be required during the first cycle of therapy and the following criteria are met: * Radiographic demonstration of clinical stability upon the completion of CNS-directed therapy and no evidence of interim progression between the completion of CNS-directed therapy and the screening radiographic study done >= 4 weeks from completion of radiotherapy and >= 2 weeks from discontinuation of corticosteroids * No stereotactic radiation or whole-brain radiation within 28 days prior to randomization
- Patients with active autoimmune disease requiring systemic corticosteroids greater than the equivalent of prednisone 10 mg daily including but not limited to: systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, colitis, vascular thrombosis associated with antiphospholipid syndrome, Wegener’s granulomatosis, Sjogren’s syndrome, Bell’s palsy, Guillain-Barre syndrome, multiple sclerosis, autoimmune thyroid disease, vasculitis, or glomerulonephritis, with the following exceptions: * Patients with a history of autoimmune hypothyroidism on a stable dose of thyroid replacement hormone are eligible * Patients with controlled type 1 diabetes mellitus on a stable insulin regimen are eligible * Patients with eczema, psoriasis, lichen simplex chronicus of vitiligo with dermatologic manifestations only who require only low potency topical steroids (e.g., hydrocortisone 2.5%, hydrocortisone butyrate 0.1%, fluocinolone 0.01%, desonide 0.05%, alclometasone dipropionate 0.05%) are eligible
- Patients receiving treatment with systemic immunosuppressive medications (including, but not limited to, prednisone, cyclophosphamide, azathioprine, methotrexate, thalidomide, and anti-tumor necrosis factor [TNF] agents) within 6 weeks must discontinue these medications prior to starting M3814 and avelumab on day 7, with the exception of: * Patients with active autoimmune disease managed with systemic corticosteroids less than the equivalent of prednisone 10 mg daily * Patients who have received acute, low dose, systemic immunosuppressant medications (e.g., a one-time dose of dexamethasone for nausea) * The use of inhaled corticosteroids and mineralocorticoids (e.g., fludrocortisone) for patients with orthostatic hypotension and adrenocortical insufficiency
- Patients who have undergone prior solid organ or bone marrow transplant with the exception of patients with prior renal transplant for whom dialysis may be employed in the event of graft rejection
- Patients with uncontrolled intercurrent illness (e.g., including but not limited to uncontrolled hypertension [HTN] [systolic blood pressure (BP) > 150, diastolic BP > 100], symptomatic congestive heart failure [CHF], unstable angina pectoris, ischemic myocardial infarction [MI] within 6 months, cardiac arrhythmia, recent transient ischemic attack [TIA or cerebrovascular accident (CVA)]) within 6 months
- Patients with serious active infection (e.g. requiring hospitalization and/or intravenous [IV] antibiotics) within 4 weeks prior to starting M3814 and avelumab, or signs/symptoms of infection or receiving oral or IV antibiotics for the treatment of active systemic infection within 2 weeks prior to starting M3814 and avelumab. Patients receiving prophylactic antibiotics are eligible
- Patients with known chronic hepatitis B virus (HBV) infection must have an undetectable viral load on suppressive therapy if indicated. Patients with known chronic hepatitis C (HCV) infection must have been treated and cured. Patients who are currently on curative treatment are eligible if they have an undetectable HCV viral load
- Patients with known human immunodeficiency virus (HIV) are allowed on study provided they have: * A stable regimen of highly active anti-retroviral therapy (HAART) * No requirement for concurrent antibiotics or antifungal agents for the prevention of opportunistic infection * A CD4 count above 250 cells/mcL * An undetectable HIV viral load on standard polymerase chain reaction (PCR)-based testing
- Patients with history of idiopathic pulmonary fibrosis, pneumonitis (including drug induced), organizing pneumonia (e.g., bronchiolitis obliterans, cryptogenic organizing pneumonia), or evidence of active pneumonitis on screening chest computed tomography (CT) scan
- Patients with known concurrent malignancy that is expected to require active treatment within two years, or may interfere with the interpretation of the efficacy and safety outcomes of this study in the opinion of the treating investigator. Superficial bladder cancer, nonmelanoma skin cancers, and low-grade prostate cancer not requiring cytotoxic therapy should not exclude participation in this trial. Patients with chronic lymphocytic leukemia (CLL) may be enrolled if they do not require active chemotherapy and their hematologic, renal and hepatic function meets criteria previously mentioned
- Patients with psychiatric illness/social situations that would limit compliance with study requirements
- History of allergic reactions attributed to compounds of similar chemical or biologic composition to M3814 or avelumab
- Patients unable to discontinue medications or substances that are potent inhibitors, inducers or sensitive substrates of CYP3A4/5 or CYP2C19 prior to starting M3814 and avelumab are ineligible. Medications or substances that are strong inhibitors of CYP3A4/5 or CYP2C19 must be discontinued at least 1 week prior to first M3814 dose. Strong inducers of CYP3A4/5 or CYP2C19 must be stopped at least 3 weeks prior to the first dose. Drugs mainly metabolized by CYP3A with a narrow therapeutic index as judged by the investigator must stop at least 1 day prior to first M3814 dose. Because the lists of these agents are constantly changing, it is important to regularly consult a frequently-updated medical reference. As part of the enrollment/informed consent procedures, the patient will be counseled on the risk of interactions with other agents, and what to do if new medications need to be prescribed or if the patient is considering a new over-the-counter medicine or herbal product. The primary elimination mechanism of avelumab is proteolytic degradation, thus there are no contraindicated medications with respect to avelumab
- Patients who cannot discontinue concomitant proton-pump inhibitors (PPIs) prior to starting M3814 and avelumab. These must be discontinued >= 5 days prior to starting M3814 and avelumab. Patients do not need to discontinue calcium carbonate. H2 blockers are allowed provided they are taken at least 2 hours after M3814 dose
- Patients receiving sorivudine or any chemically related analogues (such as brivudine) and not able to discontinue prior to starting M3814 and avelumab are excluded
- Pregnant and lactating women are excluded from this study because M3814 and avelumab are agents with the potential for teratogenic or abortifacient effects. Because there is an unknown but potential risk for adverse events in nursing infants secondary to treatment of the mother with M3814 and avelumab, breastfeeding should be discontinued if the mother is treated with M3814 and avelumab
- Patients who have received live vaccination within 30 days before starting M3814 and avelumab
Principal Investigator

Kimberly L. Johung
Sub-investigators.
- Agatha Hecht
- Alison Johnson
- Anca Bulgaru
- Andrea Brennan
- Armand Russo
- Ashita Talsania
- Benjamin Newton
- Beverly Drucker
- Clarice Grens
- D. Barry Boyd
- Elan Gorshein
- Emily Kopas
- Jacquelyne Gaddy
- Jade Vanacore
- James Vredenburgh
- Jane Kanowitz
- Jason Haldas
- Jeremy Kortmansky
- Johanna LaSala
- John Kunstman
- Jose Morales-Marin
- Justin Persico
- Karen Ann Hammond
- Katelyn Scott
- Katherine Harvey
- Kayla Martello
- Kert Sabbath
- Kristen Hoxie
- Larisa Fleysher
- Laura Sabourin
- Laura Van Metre Baum
- M. Sung Lee
- Madeline Santiago
- Matthew Austin
- Michael Burke
- Michael Cecchini
- Michael Cohenuram
- Michael Grant
- Neal Fischbach
- Osarugue Otasowie
- Pamela L. Kunz
- Pawan Karanam
- Rebecca Vanasse-Passas
- Robert Legare
- Sara Anastasio
- Sarah Carlson
- Sarah Thomen
- Sharynn Hall
- Stacey LaRosa
- Stephen Lattanzi
- Su Hsien Lim
- Susan Gueble
- Teresa White
- Victor Chang
- Vidya Kesavan
- Virginia Syombathy
- Wajih Kidwai
- Yifei Zhang
- 1-203-615-2783
- Share full article
Advertisement
Supported by
What Drugmakers Did Not Tell Volunteers in Alzheimer’s Trials
Genetic tests showed that certain patients were predisposed to brain injuries if they took the drugs. That information remained secret.

By Walt Bogdanich and Carson Kessler
By 2021, nearly 2,000 volunteers had answered the call to test an experimental Alzheimer’s drug known as BAN2401. For the drugmaker Eisai, the trial was a shot at a windfall — potentially billions of dollars — for defanging a disease that had confounded researchers for more than a century.
To assess the drug’s effectiveness and safety, Eisai sought to include people whose genetic profiles made them especially prone to develop Alzheimer’s. But these same people were also more vulnerable to brain bleeding or swelling if they received the drug.
To identify these high-risk volunteers, Eisai told everyone that they would be given a genetic test. But the results, the company added, would remain secret.
In all, 274 volunteers joined the trial without Eisai telling them they were at an especially high risk for brain injuries, documents obtained by The New York Times show.
One of them was Genevieve Lane, a 79-year-old resident of the Villages in Florida who died in September 2022 after three doses of the drug, her brain riddled with 51 microhemorrhages. An autopsy determined that the drug’s side effects had contributed to her death. Her final hours were spent thrashing so violently that nurses had to tie her down.
Another high-risk trial volunteer died, and more than 100 others suffered brain bleeding or swelling. While most of those injuries were mild and asymptomatic, some were serious and life-threatening.
“This is a medication that has some significant side effects, and we need to be aware of them,” said Dr. Matthew Schrag, the Vanderbilt University neurologist who assisted with Ms. Lane’s autopsy.
Early last year, the Food and Drug Administration approved Eisai’s Alzheimer’s drug, marketed as Leqembi, saying its modest benefit — a slight slowing of cognitive decline for a handful of months — outweighed its risks.
This past July, the agency approved a second, similar drug, Kisunla. In a clinical trial, its maker, Eli Lilly, also chose not to tell 289 volunteers that their genetic profiles made them vulnerable to brain injuries, The Times found. Dozens experienced what Lilly classified as “severe” brain bleeding.
Drug trials are in part designed to illuminate risks, which is why volunteers are routinely informed of potential dangers before joining. In both the Leqembi and Kisunla trials, volunteers first had to sign consent forms that said people with certain genetic profiles faced higher risks of brain injuries from receiving the drugs, and that participants would be tested for them — but not told the results.
Alzheimer’s experts and bioethicists expressed surprise when The Times told them about these secrecy provisions. The companies, they said, had undercut the principle of informed consent.
Calling the decision not to disclose the genetic results “certainly troubling” and “ethically fraught,” George Perry, editor of the Journal of Alzheimer’s Disease, said: “You have to ask patients if they want to know it, but then it should be disclosed. That would be part of informed consent.”
Dr. Perry added, as did several other experts, that he was unaware of similar nondisclosure provisions in other recent trials.
The secrecy provisions, which have not been previously reported, came to light as The Times investigated how the long, maddening and vastly expensive search for an effective Alzheimer’s treatment led to the testing and approval of Leqembi (and subsequently Kisunla). Reporters reviewed clinical trials, patient records and injury reports and interviewed researchers, neurologists, trial participants, families of Alzheimer’s patients, drug industry representatives and F.D.A. officials.
Leqembi and Kisunla seek to remove a misshapen protein called beta amyloid that forms plaque in the brains of patients with Alzheimer’s. To a large extent the drugs have succeeded, a remarkable scientific achievement.
Yet the drugs do not halt cognitive decline or reverse brain damage. Leqembi slows the decline for roughly five months, while Kisunla achieves a slightly longer delay. The evidence of their limited benefit has contributed to a growing realization that the dominant theory of Alzheimer’s — that sticky bands of amyloid trigger a cascade of toxic events leading to the disease — is at best incomplete and perhaps simply wrong.
At the same time, many Alzheimer’s experts worry that the new drugs’ risks have been neither fully appreciated nor understood, especially when set against their modest benefit.
“The people who are in charge of the clinical trial have not come to grips with the severity of the toxicity” of Leqembi, said Dr. Rudolph J. Castellani, a pathology professor at Northwestern’s Feinberg School of Medicine in Chicago. Dr. Castellani performed an autopsy on Jean Terrien, the other high-risk volunteer who died during the Leqembi trial.
In July, the European Union’s drug regulator recommended against approving Leqembi, co-marketed by Biogen. Last week, Australia’s regulator also declined to approve the drug. Both agencies said the drug’s temporary delay of cognitive decline did not outweigh the safety risks. In the United States, a similar conclusion was reached by the Institute for Clinical and Economic Review, a widely used independent group of analysts.
Concern about brain injuries has run through years of amyloid drug trials. In 2010, the F.D.A. recommended tightening protocols to protect the most vulnerable subjects. But researchers pushed back, and when they instead argued for broadening eligibility, the federal regulator went along.
For the Leqembi study, the secrecy provision was approved by an institutional review board run by a private-equity-backed company, Advarra. Under federal law, such boards are tasked with ensuring that trial participants do not face unnecessary risks and are informed of the studies’ risks.
While the Leqembi trial was underway, Advarra published an online “tip sheet” calling informed consent “one of the central protections” for research subjects. When asked by The Times about Advarra’s approval of the secrecy provision in the Leqembi trial, a company spokesman said he was unable to provide answers.
Eisai canceled an interview and did not respond to repeated messages over several months seeking an explanation for its decision not to disclose the genetic findings.
But a principal investigator for the Kisunla trial, Dr. David Weidman, agreed to discuss Lilly’s nondisclosure provision. He pointed to research showing that trial participants who are informed of their genetic profiles may skew their self-assessments of progress.
Dr. Weidman did not design the trial, however, and he said that in hindsight he believed bioethical concerns could have played a larger role. “Does the ethical side trump the scientific side? Personally, I would say it does,” said Dr. Weidman, a neurologist affiliated with the Banner Alzheimer’s Institute in Phoenix.
Lilly issued a statement saying that it gave participants the option to learn their genetic profiles, but only after the trial ended. “Our advice is for participants to assume they have the higher risk” at the outset, said Dr. John Sims, a Lilly neurologist who oversaw the study.
In a subsequent study of its drug, however, the company has given volunteers the option to learn their test results before entering the trial.
Eisai, in public statements about Leqembi, has cited trial findings that serious brain swelling and bleeding are rare and mostly asymptomatic.
And many researchers argue that the risk of side effects is a small price to pay for slowing, even temporarily, a devastating disease that afflicts nearly seven million Americans.
“People are robbed of everything that makes us human,” said Dr. Howard Fillit, a professor at the Icahn School of Medicine at Mount Sinai in New York and a prominent voice in Alzheimer’s research. “Can’t dress yourselves. Can’t go to the bathroom. Forget how to walk. Forget how to swallow. They’re like infants in a human body.”
‘Hope Starts Here’
Between Florida horse country to the north and Disney World to the south is a land of 4 p.m. dinners and town squares that appear more Hollywood than real, where people seek to cash in their dreams before they die. For the pharmaceutical industry, this colony, the Villages, is a petri dish of aging bodies to study in the hope of creating transformative drugs, with the prospect of almost unimaginable profits.
Recruiting people for trials with potential health risks requires skill and imagination. Charter Research runs drug trials in the Villages on behalf of pharmaceutical companies, partly by assuming the role of camp counselor, arranging a packed schedule of daily events — all free.
Over one three-week period last spring, Charter hosted 15 showings of first-run films in the Villages, as well as coffee and tea parties, karaoke, square dancing, miniature golf, pizza making, improv comedy (“laughter guaranteed”) and lunches at a Mexican restaurant, a fish house and an all-you-can-eat buffet.
One morning, as a man pushed his walker to the strains of Bruce Springsteen’s “Born to Run” blaring from speakers in the Lake Sumter Landing town square, residents filled a theater for a movie, followed by a discussion about the ravages of Alzheimer’s. The following day, Charter staked its claim in the local newspaper, with advertisements for a “New at Home Memory Test” and “Free Memory Screens.”
Genevieve Lane had come to the Villages in 2014, joining her lifelong Chicago friend Vicki Holmes. Together they had traveled the world and navigated marriages, mother-daughter conflicts and the specter of old age.
“I always said, ‘Genna, I’ll retire and find someplace in Florida, and you’ll just come live with me,’” Ms. Holmes recalled. “That was always the plan, probably, for 20 some years.”
Before her retirement, Ms. Lane had been a vice president of a transportation company. Now, she was slipping mentally. But Ms. Holmes gladly rescued her when she got lost, helped plan her daily activities and drove her to the market, hair dresser and nail salon.
Then, in 2020, Ms. Lane saw a Charter ad seeking volunteers for a trial of an experimental Alzheimer’s drug. She grew excited. Maybe the trial could help her and others, especially her children, who might someday develop the disease. Maybe this drug could make her less frustrated, more capable of enjoying life.
As Charter’s ad said: “Hope Starts Here.” Charter couldn’t promise a cure, only the hope that came with the drug known as BAN2401.
Managing the Risk
Since the 1990s, Alzheimer’s researchers had focused on proving the “amyloid-cascade hypothesis” and finding a treatment that would attack the culprit protein. Yet in trial after trial, trying to remove amyloid had produced significant side effects.
The most consequential failure involved a drug called bapineuzumab, known as bapi. Wall Street analysts predicted annual sales reaching $13 billion; one medical journal proclaimed that the future of Alzheimer’s research “may hinge on bapineuzumab’s outcome.” Those hopes dimmed in 2008 when bapi was found to cause brain injuries with little or no cognitive improvement.
Bapi’s safety issues were on the minds of F.D.A. officials in 2010 when Alzheimer’s researchers gathered in Honolulu for their annual conference. Before long, a dispatch from the federal regulator sent tremors through the conference hall. To better protect the most vulnerable patients, the F.D.A. recommended that future trials exclude volunteers with a history of microhemorrhages, ruptures of small blood vessels in the brain. At the time, people with one or two prior microhemorrhages were accepted.
Fearing this change would unduly handicap efforts to study new amyloid drugs, an ad hoc group of prominent researchers at the conference — most with close ties to the pharmaceutical industry — planned a counteroffensive. The way forward, they reasoned, lay in managing the risk, not eliminating it.
“We need to be less risk-averse in Alzheimer’s disease,” Dr. Philip Scheltens, a member of the group, would later say. “We should carefully dose up until side effects tell us to hold off.”
In the end, the group actually recommended expanding eligibility for the very people the government had hoped to protect. Now, four microhemorrhages would be allowed. The F.D.A. acquiesced, earning praise for “exemplary” collaboration from the Honolulu meeting’s sponsor, the Alzheimer’s Association, an advocacy group that accepts industry financing and arranges major conferences.
The Honolulu group took another step: It rebranded the name of the brain injuries, in part to make them sound less scary. Instead of vasogenic edema and microhemorrhages, the condition would now be called by the operatic name ARIA, an acronym for amyloid-related imaging abnormality.
But if the new guidelines opened up the trial process, they did far less to bring to fruition the industry’s quest. Over time, this search consumed so much research money that it became “too big to fail,” said Dr. Perry, the journal editor and an Alzheimer’s researcher at the University of Texas at San Antonio.
The drug maker Biogen finally broke through in June 2021, when the F.D.A. granted accelerated approval for Aduhelm, the first drug to treat Alzheimer’s purported root cause.
It proved a Pyrrhic victory. As recounted in an editorial in JAMA, the influential medical journal, Aduhelm’s approval “generated significant backlash due to unclear evidence of the drug’s clinical efficacy,” severe adverse effects and an approval process that one congressional inquiry called “rife with irregularities.” (With shrinking sales, Biogen jettisoned the drug this January.)
Eisai, though, was pinning its hopes on Leqembi.
Patients Left in the Dark
The same year as the Honolulu conference, researchers from 19 drug, biotech and medical companies came together at a Phoenix airport hotel for a highly unusual meeting. Though fierce competitors, the scientists wanted to collaborate on strategies for Alzheimer’s research — specifically whether anti-amyloid drugs could prevent Alzheimer’s in people who were still cognitively normal, before the onset of decline.
“You really needed people who might progress to cognitive impairment or start to develop symptoms of the disease in a relatively short period of time,” recalled Jessica Langbaum, a senior director at the Banner institute, which hosted the meeting.
In other words, they needed trial subjects with a gene variant called APOE4 — people with a high genetic probability of developing Alzheimer’s. People with two copies of the gene variant constitute an estimated 2 to 3 percent of the general population and 15 to 20 percent of people with Alzheimer’s. Those with only one copy make up about half of Alzheimer’s patients.
One question in Phoenix was how, or even if, these trial subjects should be told their grim genetic profiles, according to a written, contemporaneous account of the meeting.
“Researchers unfortunately have an inherent conflict of interest,” said Dr. Robert Klitzman, director of the Masters of Bioethics Program at Columbia University. “They want people to be in their study, and there are researchers who feel, if I tell people the full facts and risks, they may not want to be in the study.”
A general agreement emerged from the meeting around the importance of transparency; participants would be informed. To cope with this news, they would first undergo genetic counseling.
Subsequently, two pharmaceutical companies, Novartis and Amgen, committed to work with Banner to test an experimental drug. The trial abruptly ended in 2019 after participants experienced a “worsening in some measures of cognitive function,” according to Novartis.
Eisai took a different approach on disclosure in its Leqembi study.
In its trial protocol, the company specified that it wanted participants already experiencing mild cognitive decline. “No less than 70 percent” would have the APOE4 gene. Carriers were known to face a higher risk of brain injuries, especially those with two copies.
Before joining the trial, all volunteers had to sign a consent form. The form said they would be tested for a genetic profile that meant a higher risk of bleeding abnormalities from the drug, including cerebral microhemorrhages and brain swelling. But the form stipulated that the test results were “for research purposes” and “will not be shared with you, any insurance company, your employer, your family or any other doctor who is treating you.”
In all, the trial enrolled 957 people with one copy of the risky gene and 274 with two copies.
Dr. Marwan Sabbagh, a neurologist who advised Eisai on Leqembi, called the disclosure of genetic information “study dependent.” “Every study decides to do it a little differently,” he said.
But Arthur Caplan, a leading bioethicist at the New York University Grossman School of Medicine, said trial participants should know the dangers they face. “It’s not even a matter of ethics, it’s a matter of common sense,” he said after The Times told him about the nondisclosure clause.
An F.D.A. spokesman, Jeremy Kahn, did not address The Times’s questions about the appropriateness of the nondisclosure provision, other than to say that the agency had reviewed the trial protocol and determined it was safe.
Asked why Advarra’s institutional review board had approved the decision to keep participants in the dark, a company spokeswoman, Mel Johnson, wrote, “I’m afraid at this time I’m not going to be able to get you answers on this one.” She declined to explain why.
Institutional review boards are an outgrowth of the National Research Act of 1974, passed in response to ethical violations in clinical trials. The boards are supposed to protect the rights and welfare of human research subjects.
Originally, university-based ethics boards reviewed most drug trials, but in recent years drug companies have found it more efficient to pay a single review board to oversee trials with multiple sites. Recognizing that a profit could be made from running these boards, private equity began buying them up.
In 2021, just two private-equity-controlled companies — Advarra and WCG — reviewed 92 percent of drug trials submitted to independent I.R.B.s, according to a Government Accountability Office report last year.
The report cited concerns by some in the industry that “private-equity-backed I.R.B.s are beholden to their clients” and as a result “may be more inclined to approve a protocol and do so expediently in order to satisfy a client.”
Ms. Lane was among those in the Leqembi trial who carried two copies of APOE4. She also possessed another risk factor, the one the F.D.A. worried about when it proposed tightening eligibility requirements in 2010.
She had suffered four previous microbleeds, increasing the likelihood of brain bleeding when taking Leqembi. Eisai’s doctors apparently could not identify them, but Dr. Schrag, who assisted with Ms. Lane’s autopsy, found them when examining her pretrial brain scans.
“I’m quite confident in our interpretation,” he said. “And we published those scans so that people could contest our counts if they wanted to, and nobody has.”
Unaware of her dual risks, Ms. Lane arrived at Charter Research two days before Christmas in 2020 and signed her consent form. In the months that followed, she received only a placebo. On July 25, 2022, she agreed to participate in a new phase of the trial, in which patients could choose to receive the drug.
On Aug. 8, she had her first infusion of Leqembi.
Black Box Warnings
Nearly two years after Leqembi’s approval, several major health care institutions, including Northwestern Medicine, the Beth Israel Deaconess Medical Center in Boston and the Department of Veterans Affairs, have chosen not to give the drug to anyone with two copies of APOE4.
The drug’s rollout has been hindered by its cost — $26,500 a year — its limited efficacy and the need for frequent, expensive M.R.I. scans. More than a third of U.S. neurologists do not recommend Leqembi to Alzheimer’s patients, according to a recent report by Spherix Global Insights, a market research firm.
The F.D.A. required that Eisai include a black box warning urging physicians to consider the drug’s potential risks. Eisai now advises that “testing for APOE4 status should be performed prior to initiation of treatment,” and that prescribers should discuss the risks of ARIA with patients.
At the Alzheimer’s Association, the chief science officer, Maria C. Carrillo, believes that for many patients, Leqembi is well worth the risks.
“I think it’s transformational,” Dr. Carrillo said. “It is not a cure. We understand that. And it has side effects. So it may not be for everyone. But for those that could benefit, it offers more time during the most critical stage where you’re still independent, you still have a lot of opportunity to enjoy time with family, baptisms, weddings, graduations.”
Several experts interviewed for this article, however, argued that the risks outweighed those benefits.
In the Leqembi trial, Eisai reported that 99 people, or 39.8 percent of those carrying two copies of APOE4, experienced brain bleeding; 86 people, or 34.5 percent of those with two copies, had brain swelling. Sixteen percent of patients with one copy experienced brain bleeds.
Though typically mild and reversible, side effects such as headaches, convulsions and vision loss were sometimes so severe that patients required prolonged hospitalization and discontinuation of the therapy, according to adverse event reports sent by Eisai to the F.D.A.
Among those patients was a 70-year-old man who experienced progressive headaches, followed by a seizure, and was ultimately found to have had 61 microhemorrhages. Eisai confirmed the events were “related to study drug.” He left the study; his status was later updated to “not recovered.”
After her third dose of Leqembi, an 81-year-old woman experienced such significant brain swelling that she was withdrawn from the study. The symptoms were “related to study medication,” Eisai wrote. Over a year later, an update said she had “not recovered.” (The adverse event reports do not include APOE4 status.)
Much remains unknown about the outcomes of subjects who left the study.
Dr. Madhav Thambisetty, a neurologist and former senior investigator at the National Institute on Aging, faulted Eisai for not releasing patient-level trial data on the long-term impact of ARIA on cognition.
“I think that’s a glaring omission in my mind,” he said. “We don’t know what happened to those patients who developed serious symptoms due to ARIA,” except for two who were studied by French clinical-trial investigators. (Dr. Thambisetty recently took a job in the drug industry.)
Dr. Nicolas Villain, the French neurologist who treated the two patients, said in September that one was nonverbal and bedridden, and the other was incapacitated with severe dementia. Dr. Villain said he believed the drug’s black box warning was too weak. “These terrible events showed us that it wasn’t enough,” he said.
Some neurologists fear that brain injuries will increase now that Leqembi is widely available outside the trial, where doctors are less familiar with the drug and monitoring may not be as rigorous.
Susan Aaron, a 74-year-old retired medical coder from the Bronx, started on Leqembi this past May, according to her longtime companion, Valerie Porter. Shortly after her third infusion, Ms. Aaron, who had two copies of the APOE4 gene, was found on her couch, unconscious and drooling. She never regained consciousness. An M.R.I. showed her brain was swollen and had at least seven new microhemorrhages.
Little is understood about another potential risk of amyloid-lowering drugs — accelerated brain shrinkage.
Scott Ayton, a professor of neuroscience at the University of Melbourne, has studied the phenomenon. “The shocking results that came from our analysis,” he said in an interview, “is that these drugs, in every class that we looked at, did not preserve brain volume — they accelerate the apparent shrinkage.” He, too, criticized the drugmakers for failing to publish patient-level data to better understand that.
Brain atrophy comes naturally with aging, but it occurs faster in Alzheimer’s patients and faster yet in patients on amyloid-lowering drugs, according to neurologists.
“All of these anti-amyloid drugs have brain shrinkage associated with them — nobody’s dealt with it,” said Dr. Perry, the journal editor. “There’s no indication shrinking the brain is good.”
The Quest for a Cure Continues
More than a century after Alzheimer’s discovery and decades into drug trials, the sobering reality is that scientists still can’t agree on what causes the disease, much less how to defeat it. They don’t know what role amyloid plays in the development of Alzheimer’s or whether it is Alzheimer’s that causes the development of amyloid. Or why someone can have amyloid but not Alzheimer’s, or Alzheimer’s but no amyloid.
What a growing number of scientists say, though, is that while there’s nothing wrong with continuing to study amyloid-lowering drugs, the time has come to expand the focus.
“While beta amyloid may play a role in Alzheimer’s disease, it’s not the central disease driver, and we need a more nuanced understanding of this disease if we’re going to be successful in really moving the ball,” Dr. Schrag said.
Researchers have begun exploring other avenues, including drugs to reduce inflammation, improve blood flow and protect neurons. They are also studying repurposing drugs already F.D.A.-approved for other diseases.
Though doctors cannot yet stop or reverse cognitive decline in Alzheimer’s, there are drugs that temporarily relieve symptoms. Behavior modifications may also lower the odds of developing the disease — sleeping better, exercising more, lowering blood pressure, eating a Mediterranean diet and avoiding alcohol. Some research shows taking a multivitamin may help.
Still, the search continues, raising hope, tearing it down, then raising it again.
“It’s probably the most feared disease I know of, even more than terminal cancer,” said Dr. Caplan, the bioethicist. “It’s disintegration of self, loss of dignity.”
As much as ethicists love informed consent, he said, Alzheimer’s fuels a desperation that can lead people to say, “Yes, I’ll take any life preserver you could throw in the water, even if it’s leaky. I don’t care. Give me something.”
An Autopsy ‘Unlike Anything’
When Eisai tossed a life preserver, Genevieve Lane reached out to grab it. So did Jean Terrien.
Ms. Terrien had practiced law in Washington and advocated “sensible” gun control, according to her obituary. After her son was born, she quit law and became a psychotherapist. After three infusions of Leqembi, she died of complications from a stroke. She was 65.
Scanned images showed widespread bleeding that Dr. Castellani, the Northwestern physician who performed Ms. Terrien’s autopsy, described as “quite unlike anything I really encountered across the spectrum of human illnesses.”
Ms. Lane developed a headache after her first infusion. It happened again after her second, except this time she stayed in bed for hours. Another headache followed her third infusion, but she and her friend, Ms. Holmes, went to dinner at Takis Greek Italian Restaurant, their favorite in the Villages.
At 5:30 p.m., while waiting for food, Ms. Lane slumped and became unresponsive. She died at a hospital five days later.
On the day Ms. Lane died, while she was still on life support, Charter Research called Ms. Holmes to remind her that her friend was scheduled for another infusion.
“‘Are you not aware that she’s in the hospital?’” Ms. Holmes recalled saying. “‘We’re talking about pulling the plug.’ I said, ‘No, she won’t be coming back anytime — ever.’”
An earlier version of this article included a description of a data analysis by a group of leading researchers that suggested that patients taking Leqembi and an earlier drug, Aduhelm, had a higher mortality rate than untreated patients of a similar age in the United States. The Times removed references to the analysis after it was withdrawn amid objections from several of its authors that it had been posted online prematurely, given the limitations of the data.
Walt Bogdanich joined The Times in 2001 as investigative editor for the Business desk. Since 2003, he has worked as an investigative reporter. He has won three Pulitzer Prizes. More about Walt Bogdanich
Carson Kessler is an investigative reporter and a member of the 2023-24 Times Fellowship class, a program for journalists early in their career. More about Carson Kessler
The Fight Against Alzheimer’s Disease
Alzheimer’s is the most common form of dementia, but much remains unknown about this daunting disease..
The shifts in behavior that are experienced by people with dementia can be challenging for caregivers to manage. Experts have five strategies that can help .
In clinical trials for Alzheimer’s drugs, genetic tests showed that certain patients were predisposed to brain injuries if they took the drugs. That information remained secret .
Memory loss isn’t the only sign of dementia. Here are five other common red flags to look out for .
How is Alzheimer’s diagnosed? What causes Alzheimer’s? We answered some common questions .
Scientists have made another major stride toward the long-sought goal of diagnosing Alzheimer’s with a simple blood test .
Clinical Trials Administration
Specialized Certificate
About the Clinical Trials Administration Program
- managers-in-training and others who want to refine or update skills
- research associates and coordinators
- biomedical and research scientists
- nurses and allied health professionals
- statisticians and database administrators
- international clinical trials professionals
- individuals with degrees in science, psychology, or related areas whom are entering the field
Certificate Benefits
- Offers a foundation in professional principles upon which clinical trials are based
- Provides opportunity to develop, practice, and apply skills most beneficial on-the-job
- Builds skills to assume more responsible roles within the industry
- Offers an opportunity to demonstrate working industry knowledge
- Provides networking opportunities with instructors and peers
Target Audience
The curriculum is intended for
- managers in training and others who want to refine or update skills
- individuals with degrees in science, psychology or related areas who are entering the field
- anyone interested in the dynamic clinical trials field.
How to Apply
This certificate requires an application before taking any courses. There will be a $0.00 fee to apply to this program. Students will be required to pay a $95.00 fee upon acceptance to start their certificate program.
Get More Information
For detailed information, please contact our Healthcare & Behavioral Sciences department.
- 858-534-9262
- [email protected]
Required Courses
Clinical trials administration intensive.
Units: 4.00
This five day intensive course provides a solid foundation in the principles upon which clinical trials are based. Topics include: drug development process, device development, GCPs (ICH and FDA), Case Report Form design, investigator selection, IRBs/informed consent, trial design & protocol, site and study management, monitoring, regulatory issues, and data analysis.
(FPM 40272 FOLLOWS FPM 40273)
Clinical trials administration program.
Units: 7.00
Prerequisite: Intensive Workshop, FPM 40273. How the Program Works While pursuing the Specialized Certificate in Clinical Trials Administration, you must successfully complete the following three sections: Section One Clinical Trials Intensive Workshop (5 days, 40 hours, 4 units) Section Two Required Courses Offered Online: Nuts & Bolts of Monitoring Clinical Trials (2 units) Setting Up a New Clinical Study (2 units) Working with Clinical Research Organizations (CROs) (1 unit) Section Three Clinical Trials Follow-on Workshop. The Final Session (2 days, 16 hours, 2 units). Participation in...
Demand and Job Prospects
Certificate graduates fulfill educational minimums for different entry level positions (Clinical Trial Assistant, Clinical Research Coordinator, Data Entry Associate) in pharmaceutical and medical device companies (sponsors), Clinical Research Organizations (CROs), or clinical sites such as large hospitals and clinics.
U.S. Department of Labor projections show that these types of occupations are forecast to grow “much faster than average” through 2024. Depending on individual’s experience and highest level of education completed salaries can range between $16-33 per hour.
Conditions for Admission
Please see the application for specific admission requirements for this program. Although a college degree or professional experience in the field are not required, applicants with such experience may receive preference for admission when program capacity is limited.
Certificate Guidelines
Part 1: Clinical Trials Administration Intensive, FPM 40273, offered Spring and Fall quarters. This is followed by:
Part 2: Follow-On Program, FPM 40272; package includes 3 online classes and the Capstone workshop (also available via distance learning for students residing out of region) for one fee. Must Take FPM 40273 before FPM 40272.
Special Notes
Continuing Education hours awarded: 40 hours for the Clinical Trials Administration Intensive; an additional 70 hours for the "package" of three online courses and the Follow-On Workshop. Applicable to ACRP and SoCRA education hours requirements.
Military spouses are eligible to use My CAA $4000 scholarship. Apply at MyCAA.

Advisory Board
Jan agee, bs, ccra, barbra laplante, ma, jenny paredes, ba, ma, phd, related programs.

Biotechnology Project Management

Clinical Trials Design and Management

Quality Assurance and Control
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials
Subash c gupta, sridevi patchva, bharat b aggarwal.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Received 2012 Aug 16; Accepted 2012 Oct 20; Collection date 2013 Jan.
Extensive research over the past half century has shown that curcumin (diferuloylmethane), a component of the golden spice turmeric ( Curcuma longa ), can modulate multiple cell signaling pathways. Extensive clinical trials over the past quarter century have addressed the pharmacokinetics, safety, and efficacy of this nutraceutical against numerous diseases in humans. Some promising effects have been observed in patients with various pro-inflammatory diseases including cancer, cardiovascular disease, arthritis, uveitis, ulcerative proctitis, Crohn’s disease, ulcerative colitis, irritable bowel disease, tropical pancreatitis, peptic ulcer, gastric ulcer, idiopathic orbital inflammatory pseudotumor, oral lichen planus, gastric inflammation, vitiligo, psoriasis, acute coronary syndrome, atherosclerosis, diabetes, diabetic nephropathy, diabetic microangiopathy, lupus nephritis, renal conditions, acquired immunodeficiency syndrome, β-thalassemia, biliary dyskinesia, Dejerine-Sottas disease, cholecystitis, and chronic bacterial prostatitis. Curcumin has also shown protection against hepatic conditions, chronic arsenic exposure, and alcohol intoxication. Dose-escalating studies have indicated the safety of curcumin at doses as high as 12 g/day over 3 months. Curcumin’s pleiotropic activities emanate from its ability to modulate numerous signaling molecules such as pro-inflammatory cytokines, apoptotic proteins, NF–κB, cyclooxygenase-2, 5-LOX, STAT3, C-reactive protein, prostaglandin E 2 , prostate-specific antigen, adhesion molecules, phosphorylase kinase, transforming growth factor-β, triglyceride, ET-1, creatinine, HO-1, AST, and ALT in human participants. In clinical trials, curcumin has been used either alone or in combination with other agents. Various formulations of curcumin, including nanoparticles, liposomal encapsulation, emulsions, capsules, tablets, and powder, have been examined. In this review, we discuss in detail the various human diseases in which the effect of curcumin has been investigated.
Key words: clinical trial, curcumin, human diseases, inflammation, safety
INTRODUCTION
Despite considerable efforts, the prevalences of complex multigenic human diseases such as cardiovascular diseases, metabolic diseases, cancer, and neurological diseases have not decreased significantly in recent years. A number of monotargeted “smart” drugs have emerged over the past decade; however, the aforementioned diseases are caused by perturbations of multiple signaling pathways. Thus, attacking only one of these multiple pathways is highly unlikely to be effective ( 1 , 2 ). In addition, such monotargeted “smart” drugs are often very expensive and can produce numerous adverse effects. These features of monotargeted drugs underscore the importance of multitargeted, innocuous, inexpensive, and readily available dietary agents or nutraceuticals for the prevention and treatment of human diseases. Curcumin is one such widely studied nutraceutical that was first discovered about two centuries ago by Harvard College laboratory scientists Vogel and Pelletier from the rhizomes of Curcuma longa (turmeric) ( 3 , 4 ).
Curcumin is a highly pleiotropic molecule that was first shown to exhibit antibacterial activity in 1949 ( 5 ). Since then, this polyphenol has been shown to possess anti-inflammatory, hypoglycemic, antioxidant, wound-healing, and antimicrobial activities ( 6 ). Extensive preclinical studies over the past three decades have indicated curcumin’s therapeutic potential against a wide range of human diseases ( 7 ). In addition, curcumin has been shown to directly interact with numerous signaling molecules ( 8 ). These preclinical studies have formed a solid basis for evaluating curcumin’s efficacy in clinical trials.
Although the therapeutic use of Curcuma was recorded as early as 1748 ( 9 ), the first article referring to the use of curcumin in human disease was published in 1937 by Oppenheimer ( 10 ). In this study, the author examined the effects of “curcumen” or “curcunat” containing 0.1 g to 0.25 g sodium curcumin and 0.1 g calcium cholate in human biliary diseases. An intravenous injection of 5% sodium curcumin solution in healthy persons was associated with rapid emptying of the gallbladder. The author treated 67 patients with subacute, recurrent, or chronic cholecystitis. Oral administration of curcunat for 3 weeks showed remarkably good results against cholecystitis. All but one patient were completely cured of the disease throughout periods of observation lasting from 3 months to more than 3 years. No ill effects were observed or reported, even when the medication was continued for many consecutive months ( 10 ). Since this initial identification, interest in curcumin research in human participants has increased remarkably (Fig. 1a ). As of July 2012, observations from almost 67 clinical trials have been published, whereas another 35 clinical trials are in progress.
a The interest in curcumin research in human participants has increased remarkably over the years. b Human diseases against which curcumin has exhibited activity
The safety, tolerability, and nontoxicity of curcumin at high doses are well established by human clinical trials ( 3 , 4 ). Our own group found that curcumin at 8 g/day in combination with gemcitabine was safe and well-tolerated in patients with pancreatic cancer ( 11 , 12 ). The clinical trials conducted thus far have indicated the therapeutic potential of curcumin against a wide range of human diseases. It has also shown protection against hepatic conditions, chronic arsenic exposure, and alcohol intoxication (Fig. 1b ). In these clinical trials, curcumin has been used either alone or in combination with other agents such as quercetin, gemcitabine, piperine, docetaxel, soy isoflavones, bioperine, sulfasalazine, mesalamine, prednisone, lactoferrin, N -acetylcysteine, and pantoprazole (Table I ).
Completed Clinical Trials with Curcumin
8-OHdG 8-hydroxydeoxyguanosine, ACF aberrant crypt foci, As arsenic, ATT antituberculosis treatment, CDAI Crohn disease activity index, CD4 cluster of differentiation 4, GSH glutathione, HDL high-density lipoprotein, H. pylori Helicobacter pylori , HOMA homeostasis model assessment, IKK IκB kinase, IL interleukin, IR insulin resistance, LDL low-density lipoprotein, M 1 G pyrimido[1,2- a ]purin-10( 3H )-one, MAPK mitogen-activated protein kinase, MDA malondialdehyde, MMP-3 matrix metalloproteinase-3, NB-UVB narrowband UVB, PGE 2 prostaglandin E 2 , PhK phosphorylase kinase, PSA prostate-specific antigen, RA rheumatoid arthritis, STE standard turmeric extract, TGF-β transforming growth factor beta, TNF-α tumor necrosis factor-α
a Combination study
b Study with curcumin analogue
c Study with turmeric/ C . longa
How a single agent can possess these diverse effects has been an enigma over the years, both for basic scientists and clinicians. However, numerous lines of evidence have indicated curcumin’s ability in human participants to modulate multiple cell signaling molecules such as pro-inflammatory cytokines (tumor necrosis factor [TNF]-α, interleukin [IL]-1β, IL-6), apoptotic proteins, NF–κB, cyclooxygenase (COX)-2, STAT3, IKKβ, endothelin-1, malondialdehyde (MDA), C-reactive protein (CRP), prostaglandin E 2 , GST, PSA, VCAM1, glutathione (GSH), pepsinogen, phosphorylase kinase (PhK), transferrin receptor, total cholesterol, transforming growth factor (TGF)-β, triglyceride, creatinine, HO-1, antioxidants, AST, and ALT (Table II ).
Molecular Targets of Curcumin in Human Participants
↓, Downregulation;↑, upregulation
8-OHdG 8-hydroxydeoxyguanosine, ALT alanine transaminase, AST aspartate transaminase, Bax Bcl-2–associated X protein, Bcl-2 B cell lymphoma-2, CD cluster of differentiation, CDAI Crohn disease activity index, COX-2 cyclooxygenase 2, CRP C-reactive protein, ET-1 endothelin-1, ESR erythrocyte sedimentation rate, GSH glutathione, GST glutathione S-transferase, GPX glutathione peroxidase, HDL high-density lipoprotein, HO-1 hemoxygenase-1, H. pylori Helicobacter pylori , HOMA homeostasis model assessment, IL interleukin, IR insulin resistance, LDL low-density lipoprotein, MAPK mitogen-activated protein kinase, MDA malondialdehyde, M 1 G pyrimido[1,2- a ]purin-10( 3H )-one, MMP-3 matrix metalloproteinase-3, NF – κB nuclear factor kappa-light-chain-enhancer of activated B cells, NTBI non-transferrin bound iron, NTT N-telopeptide of type 1 collagen, PGE 2 prostaglandin E 2 , PhK phosphorylase kinase, PSA prostate-specific antigen, pSTAT3 phosphorylated form of signal transducer and activator of transcription 3, ROS reactive oxygen species, sCD40L soluble cluster of differentiation 40 ligand, SOD superoxide dismutase, sPG I serum pepsinogen I, sPG II serum pepsinogen II, sVCAM soluble vascular cell adhesion molecule, TC total cholesterol, TG triglyceride, TNF-α tumor necrosis factor-α, TRR transferrin receptor
Although curcumin has shown efficacy against numerous human ailments, poor bioavailability due to poor absorption, rapid metabolism, and rapid systemic elimination have been shown to limit its therapeutic efficacy ( 75 ). As a result, numerous efforts have been made to improve curcumin’s bioavailability by altering these features. The use of adjuvants that can block the metabolic pathway of curcumin is the most common strategy for increasing the bioavailability of curcumin. The effect of combining piperine, a known inhibitor of hepatic and intestinal glucuronidation, was evaluated on the bioavailability of curcumin in healthy human volunteers ( 76 ). In humans receiving a dose of 2 g of curcumin alone, serum levels of curcumin were either undetectable or very low. Concomitant administration of 20 mg of piperine with curcumin, however, produced much higher concentrations within 30 min to 1 h after drug treatment; piperine increased the bioavailability of curcumin by 2,000%. Other promising approaches to increase the bioavailability of curcumin in humans include the use of nanoparticles ( 73 ), liposomes ( 77 ), phospholipid complexes ( 78 ), and structural analogues ( 75 ). Meriva is a patented phytosome complex of curcumin with soy phosphatidylcholine that has better bioavailability than curcumin. The absorption of a curcuminoid mixture and Meriva was examined in a randomized, double-blind, crossover human study ( 78 ). Total curcuminoid absorption was about 29-fold higher for the Meriva mixture than it was for the corresponding unformulated curcuminoid mixture. Interestingly, the phospholipid formulation increased the absorption of demethoxylatedcurcuminoids much more than that of curcumin ( 78 ). The bioavailability of curcumin has also been shown to be greatly enhanced by reconstituting curcumin with the non-curcuminoid components of turmeric ( 79 ).
Most of the curcumin’s clinical studies have been focused mainly on people with health problems. A recent study, however, evaluated the health-promoting efficacy of lipidated curcumin in healthy middle aged participants (40–60 years old). In this study, the participants were given either lipidated curcumin (80 mg/day) or placebo for 4 weeks. Curcumin, but not placebo, produced decrease in salivary amylase and in the plasma levels of triglycerides, beta amyloid, alanine amino transferase, and sICAM. Furthermore, curcumin administration in these participants increased salivary radical scavenging capacities and activities in plasma catalase, myeloperoxidase, and nitric oxide production. Overall, these results demonstrated the health-promoting effects of lipidated curcumin in healthy middle aged people ( 80 ).
Although relatively pure curcumin has been used in some human studies, most studies have used either a mixture of curcuminoids or even turmeric, from which curcuminoids are derived. Approximately 2%–6% ( w/w ) of turmeric is curcuminoids. The latter contains 80% curcumin, 18% demethoxycurcumin, and 2% bisdemethoxycurcumin. The United States Food and Drug Administration has approved curcumin as being GRAS (generally recognized as safe), and the polyphenol is now being used as a supplement in several countries ( 81 ). It is marketed in several forms, including capsules, tablets, ointments, energy drinks, soaps, and cosmetics. In the following sections, we summarize the studies documenting the activities of curcumin against numerous diseases in human participants and its mechanisms of action.
COMPLETED CLINICAL TRIALS
Cancer therapy.
Cancer is a multistage process involving a series of events and resulting from the dysregulation of more than 500 genes at multiple steps in cell signaling pathways ( 82 ). Although currently available monotargeted cancer therapeutics have had some effect, these drugs are associated with numerous adverse effects and are expensive. The current paradigm for cancer treatment is either to combine several monotargeted drugs or to design drugs that modulate multiple targets. Because of its multitargeting activities, curcumin has exhibited activities against numerous cancer types in human clinical trials.
Probably the first indication of curcumin’s anticancer activities in human participants was shown in 1987 by Kuttan and co-workers ( 26 ), who conducted a clinical trial involving 62 patients with external cancerous lesions. Topical curcumin was found to produce remarkable symptomatic relief as evidenced by reductions in smell, itching, lesion size, and pain. Although the effect continued for several months in many patients, only one patient had an adverse reaction ( 26 ). Since then, curcumin, either alone or in combination with other agents, has demonstrated potential against colorectal cancer, pancreatic cancer, breast cancer, prostate cancer, multiple myeloma, lung cancer, oral cancer, and head and neck squamous cell carcinoma (HNSCC).
Colorectal Cancer
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States, with 143,460 new cases and 51,690 deaths expected in 2012. Currently, there is no effective treatment except resection at a very early stage with or without chemotherapy. Thus, new strategies are needed to replace or complement current therapies. Curcumin has demonstrated potential against CRC in numerous clinical trials.
A dose-escalation pilot study evaluated the pharmacokinetics and pharmacodynamics of a standardized Curcuma extract in proprietary capsule form at doses between 440 and 2,200 mg/day, containing 36–180 mg of curcumin ( 13 ). Fifteen patients with advanced CRC refractory to standard chemotherapies received Curcuma extract daily for up to 4 months. Activity of glutathione S-transferase and levels of M 1 G, a marker of DNA adduct formation, were measured in patients’ blood cells. Oral Curcuma extract was well-tolerated, and dose-limiting toxicity was not observed. Neither curcumin nor its metabolites were detected in blood or urine, but curcumin was recovered from feces. Curcumin sulfate was identified in the feces of one patient. Ingestion of 440 mg of Curcuma extract containing 36 mg of curcumin for 29 days was accompanied by a 59% decrease in lymphocytic glutathione S-transferase activity. At higher dose levels, however, the effect was not observed. Leukocytic M 1 G levels were constant within each patient and unaffected by treatment ( 13 ).
In another dose-escalation study that explored the pharmacology of curcumin in humans ( 14 ), 15 patients with advanced CRC refractory to standard chemotherapies consumed capsules compatible with curcumin doses of between 0.45 and 3.6 g/day for up to 4 months. Levels of curcumin and its metabolites in plasma, urine, and feces were analyzed. Curcumin and its glucuronide and sulfate metabolites were detected in plasma in the 10 nmol/L range and in urine. A daily dose of 3.6 g of curcumin caused 62% and 57% decrease in inducible prostaglandin E 2 production in blood samples taken 1 h after the dose was administered on days 1 and 29, respectively. A daily oral dose of 3.6 g of curcumin was recommended for the phase II evaluation in the prevention or treatment of cancers outside the gastrointestinal tract ( 14 ).
In another study, patients were given curcumin capsules at three different doses (3.6, 1.8, and 0.45 g/day) for 7 days ( 15 ). The recoveries of curcumin in normal and malignant colorectal tissues of patients receiving 3.6 g of curcumin were 12.7 ± 5.7 and 7.7 ± 1.8 nmol/g, respectively. In addition, two metabolites of curcumin, curcumin sulfate and curcumin glucuronide, were identified in the tissue samples. Trace levels of curcumin were found in the peripheral circulation. The levels of M 1 G were also decreased by curcumin treatment in malignant colorectal tissue. However, levels of COX-2 were unaffected by curcumin. The study concluded that a daily dose of 3.6 g of curcumin is pharmacologically efficacious in CRC patients ( 15 ).
Curcumin has also demonstrated potential for the prevention and treatment of CRC in combination with other agents. Familial adenomatous polyposis (FAP) is an autosomal-dominant disorder characterized by hundreds of colorectal adenomas that eventually develop into CRC. Although nonsteroidal anti-inflammatory drugs (NSAIDs) and COX-2 inhibitors have been shown to reduce the adenomas in this syndrome, these drugs produce numerous adverse effects. One study evaluated whether the combination of curcumin and quercetin could suppress adenomas in patients with FAP ( 16 ). Five patients with FAP who had undergone prior colectomy received combinations of curcumin (480 mg) and quercetin (20 mg) orally three times a day, and the number and size of polyps were assessed at baseline and after therapy. The number and size of polyps had decreased after 6 months of combination treatment without any appreciable toxicity in the five patients (Fig. 2a ). Although the combinations seemed to reduce the adenomas, randomized controlled trials are needed to further validate these findings ( 16 ).
a Effects of curcumin and quercetin on polyp number and polyp size in patients with familial adenomatous polyposis [reprinted from Clinical Gastroenterology and Hepatology , vol 4, Cruz–Correa et al. , Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis, 1035–1038, copyright (2006), with permission from Elsevier ( 16 )]. b MDA and GSH levels in patients with tropical pancreatitis after oral administration of curcumin for 6 weeks [reprinted by permission from Indian Journal of Medical Research , vol 122, issue 4, pages 315–318, Durgaprasad et al. , copyright (2005) the IJMR ( 19 )]. GSH , glutathione; MDA , malondialdehyde
In a nonrandomized, open-label clinical trial in smokers, polyphenol reduced the formation of aberrant crypt foci (ACF), the precursor of colorectal polyps ( 17 ). In this study, 44 smokers were given curcumin orally at two different doses (2 or 4 g/day) for 30 days. The levels of procarcinogenic eicosanoids, prostaglandin E 2 , and 5-hydroxyeicosatetraenoic acid in ACF or normal flat mucosa were unaffected by the curcumin treatment at lower doses. Curcumin at 4 g/day, however, significantly reduced ACF formation. The reduction in ACF formation by curcumin was associated with a significant fivefold increase in post-treatment plasma curcumin/conjugate levels. Curcumin was well-tolerated at both concentrations. These findings demonstrated the effect of curcumin against ACF formation in smokers ( 17 ). However, if the mechanism by which curcumin reduces ACF formation can be identified, it might further strengthen curcumin’s utility as a cancer chemopreventive agent.
In another recent study, curcumin was administered to patients with CRC after diagnosis and before surgery ( 18 ). Curcumin (360 mg in a capsule form) was given three times a day for 10–30 days. Curcumin administration increased body weight, decreased serum TNF-α level, increased the number of apoptotic cells, and enhanced the expression of p53 in tumor tissue. The authors of this study concluded that curcumin treatment can improve the general health of CRC patients via the mechanism of increased p53 expression in tumor cells ( 18 ). However, such a correlation does not necessarily mean that p53 induction by curcumin can improve the general health of patients with CRC. Further studies are necessary to confirm these claims.
In summary, the studies discussed in this section suggest curcumin’s safety and efficacy in patients with CRC. Larger randomized and well-controlled clinical trials will further confirm curcumin’s clinical efficacy against CRC.
Pancreatic Cancer
Pancreatic cancer is the fourth most common cause of cancer death across the globe ( 83 ). It often develops without early symptoms and is diagnosed at an advanced stage. Tropical pancreatitis is a type of chronic pancreatitis common in tropical populations. If the disease persists longer, patients with tropical pancreatitis may develop pancreatic cancer. Because oxidative stress is believed to be one of the causes of tropical pancreatitis, use of antioxidants may improve this condition. A single-blind, randomized, placebo-controlled study from India was conducted to evaluate the effects of oral curcumin with piperine on the pain and markers associated with oxidative stress in patients with tropical pancreatitis ( 19 ). Twenty patients with tropical pancreatitis were randomly assigned to receive 500 mg of curcumin with 5 mg of piperine or to receive placebo for 6 weeks, and the effects on the pattern of pain and on red blood cell (RBC) levels of MDA and GSH were assessed. The results indicated a significant reduction in the erythrocyte MDA levels compared with placebo after curcumin therapy, with a significant increase in GSH levels (Fig. 2b ). The pain, however, was not improved by curcumin administration. The authors of this study concluded that oral curcumin with piperine may reverse lipid peroxidation in patients with tropical pancreatitis ( 19 ).
Curcumin was found safe and well-tolerated in a phase II clinical trial of patients with advanced pancreatic cancer ( 12 ). Of the 25 patients enrolled in the study, 21 were evaluable for response. Patients were given 8 g of curcumin per day orally until disease progression, with restaging every 2 months. Circulating curcumin was detectable as the glucuronide and sulfate conjugate forms, albeit at low steady-state levels, suggesting poor oral bioavailability. Two patients showed clinical biological activity, and one had ongoing stable disease for more than 18 months. Interestingly, one additional patient had a brief, but marked, tumor regression accompanied by significant increases in serum cytokine levels (IL-6, IL-8, IL-10, and IL-1 receptor antagonists). No toxicities associated with curcumin administration were noted in the patients. A downregulation in the expression of NF–κB, COX-2, and pSTAT3 in peripheral blood mononuclear cells of patients was observed after curcumin intake. There was considerable interpatient variation in plasma curcumin levels, and drug levels peaked at 22 to 41 ng/ml and remained relatively constant over the first 4 weeks. The study concluded that the oral curcumin is well-tolerated and, despite limited absorption, has biological activity in some patients with pancreatic cancer ( 12 ).
An open-label phase II trial evaluated the efficacy of curcumin in combination with gemcitabine against advanced pancreatic cancer ( 20 ). Seventeen patients enrolled in the study received 8 g of curcumin orally per day for 4 weeks; gemcitabine was given concurrently at an intravenous dose of 1,000 mg/m 2 three times a week. Eleven patients were eligible for evaluation of the efficacy of this combination since curcumin or the whole treatment was discontinued very early due to toxicity in five patients and sudden death in one patient. One of the 11 evaluable patients (9%) showed a partial response; four (36%) had stable disease, and six (55%) had tumor progression. Time to tumor progression was 1–12 months (median, 2.5 months), and overall survival was 1–24 months (median, 5 months). The authors of this study concluded that a curcumin dose of 8 g/day is above the maximum tolerated dose when taken with gemcitabine and that the efficacy of the combinations seemed modest. A large number of patients are needed to draw a solid conclusion ( 20 ). Kanai et al. recently evaluated the safety and feasibility of combinations of curcumin and gemcitabine in 21 patients with gemcitabine-resistant pancreatic cancer. Curcumin at 8 g/day in combination with gemcitabine was safe and well-tolerated ( 11 ).
Breast Cancer
Breast cancer is the second most common cause of cancer death in women and is very rare in men. According to one estimate, almost 226,870 new cases of invasive breast cancer are expected to occur among women in the United States during 2012. Docetaxel, a microtubule inhibitor, has been commonly used either as a single agent in metastatic disease or in combination with other chemotherapeutic agents in early stages of breast cancer. The feasibility and tolerability of the combination of docetaxel and curcumin in patients with advanced and metastatic breast cancer were evaluated in an open-label phase I trial ( 21 ). Fourteen patients with advanced or metastatic breast cancer were enrolled in the study. Docetaxel (100 mg/m 2 ) was administered as a 1-h intravenous infusion every 3 weeks on day 1 for six cycles. Curcumin was given orally from 0.5 g/day for seven consecutive days by cycle (from day−4 to day+2) and escalated until a dose-limiting toxicity occurred. The primary endpoint was to determine the maximal tolerable dose of the combination of dose-escalating curcumin and the standard dose of docetaxel chemotherapy in advanced and metastatic breast cancer patients. Secondary objectives included toxicity, safety, vascular endothelial growth factor and tumor markers measurements, and assessment of objective and clinical responses to the combination therapy. The maximum tolerable dose of curcumin was found to be 8 g/day, whereas the recommended dose was 6 g/day for seven consecutive days every 3 weeks in combination with a standard dose of docetaxel ( 21 ).
Prostate Cancer
Prostate cancer is the most common malignancy of men. According to the American Cancer Society’s most recent estimates, 241,740 new cases of prostate cancer will occur in the United States during 2012. The disease is normally monitored by the prostate-specific antigen (PSA) test. An elevated level of PSA per se reflects the risk of developing prostate cancer. Thus, intervention to improve the PSA level may help prevent prostate cancer. A randomized, double-blind, controlled study evaluated the effects of soy isoflavones and curcumin on serum PSA levels in men who underwent prostate biopsies because of increased PSA but who had negative findings for prostate cancer ( 22 ). Eighty-five participants were randomly assigned to take a supplement containing isoflavones and curcumin or placebo daily. Participants were subdivided by the cut-off of their baseline PSA value at 10 ng/ml. Forty-three participants were given a combination of 100 mg of curcumin and 40 mg of isoflavones, and 42 were given placebo for 6 months. PSA values were evaluated before and 6 months after treatment. PSA levels decreased in the patient group, with PSA values greater than10 ng/ml among those who received supplementation containing isoflavones and curcumin (Fig. 3a ). These results indicated that isoflavones and curcumin could modulate serum PSA levels. The authors of this study concluded that curcumin presumably synergizes with isoflavones to suppress PSA production ( 22 ).
a Serum PSA levels at the baseline (pre) and after administration of isoflavones (40 mg/day) and curcumin (100 mg/day) supplements or placebo (post) for 6 months in participants with PSA < 10 or PSA ≥10 [reprinted with permission from Ide et al. , (2010), Prostate, John Wiley and Sons ( 22 )]. b Effects of turmeric extract, turmeric oil, and turmeric oleoresin on micronuclei formation in exfoliated buccal mucosal cells of patients with oral submucous fibrosis [reprinted from Cancer Letters , vol 116, Hastak et al. , Effect of turmeric oil and turmeric oleoresin on cytogenetic damage in patients suffering from oral submucous fibrosis, pages 265–269, copyright (1997), with permission from Elsevier ( 27 )]. PSA , prostate-specific antigen
Multiple Myeloma
Multiple myeloma, also known as plasma cell myeloma, is a generalized malignancy of plasma cells associated with diverse clinical features, including bone lesions, hypercalcemia, anemia, and renal failure. It is the second most common hematological cancer in the United States after non-Hodgkin lymphoma. While advances in treatment, including the use of bortezomib (Velcade), thalidomide, and lenalidomide (Revlimid), have improved patient outcomes, multiple myeloma remains an incurable disease for most patients.
Monoclonal gammopathy of undetermined significance (MGUS) is a common premalignant plasma cell proliferative disorder with a lifelong risk of progression to multiple myeloma. The disease is characterized by a serum M-protein value of <30 g/L, fewer than 10% plasma cells in the bone marrow, no or a low amount of M protein in the urine, absence of lytic bone lesions, anemia, and renal insufficiency ( 84 ). Golombick et al. ( 23 ) conducted a single-blind, crossover pilot study to determine the effects of curcumin on plasma cells and osteoclasts in patients with MGUS. Twenty-six patients with MGUS who enrolled in this study were randomly assigned to two groups. In group 1, 17 patients were given curcumin at the study start and were then crossed over to placebo after 3 months. In group 2, nine patients were given placebo initially and then crossed over to curcumin. Curcumin decreased the paraprotein load in the ten patients with paraprotein >20 g/L, and five of these ten had a 12% to 30% reduction in paraprotein levels while receiving curcumin therapy. In addition, 27% of patients receiving curcumin had a >25% decrease in urinary N -telopeptide of type I collagen ( 23 ). The study suggested the therapeutic potential of curcumin against MGUS.
Vadhan-Raj et al. ( 24 ) evaluated the safety, tolerability, and clinical efficacy of curcumin in 29 patients with asymptomatic, relapsed, or plateau phase multiple myeloma. Curcumin was given either alone (orally at 2, 4, 6, 8, or 12 g/day in two divided doses) or in combination with bioperine (10 mg in two divided doses) for 12 weeks. Curcumin and bioperine were well-tolerated, with no significant adverse events. Of the 29 evaluable patients, 12 continued treatment for more than 12 weeks, and five patients (one at a dose of 4 g, two at 6 g, and two at 8 g) completed a full year of treatment with stable disease. Peripheral blood mononuclear cells from 28 patients examined at baseline showed constitutively active NF–κB, COX-2, and STAT3. Furthermore, oral administration of curcumin was associated with significant downregulation in the constitutive activation of NF–κB and STAT3, and it suppressed COX-2 expression in most of the patients. These observations suggest the potential of curcumin against multiple myeloma ( 24 ); however, well-controlled clinical trials with larger number of patients are required to confirm the efficacy of curcumin against multiple myeloma.
Lung Cancer
Smokers excrete significant amounts of mutagens in the urine and are at high risk of developing lung cancer. Whereas smoking increases the risk for mutagenicity and lung cancer, dietary factors including turmeric reduce the risk. One study assessed the anti-mutagenic effects of turmeric in 16 chronic smokers and six non-smokers who served as a control ( 25 ). When given at 1.5 g/day for 30 days, turmeric significantly reduced the urinary excretion of mutagens in the smokers, but in the control group, no changes in the urinary excretion of mutagens were observed. Furthermore, turmeric had no significant effect on serum aspartate aminotransferase and alanine aminotransferase, blood glucose, creatinine, or lipid profile ( 25 ). Authors of this study suggested that dietary turmeric can act as an effective anti-mutagen in smokers and can reduce the risk of lung cancer.
Cancer Lesions
Oral cancer is one of the leading cancers of the Indian subcontinent and is associated mostly with tobacco chewing. The most common precancerous oral lesions such as oral submucous fibrosis, oral leukoplakia, and oral lichen planus are associated with tobacco chewing. In addition to tobacco chewing, numerous other factors contribute to the onset of oral lichen planus including the use of NSAIDs, sulfonylureas, anti-malarials, and β-blockers as well as genetic factors and stress conditions ( 85 ). The patients experiencing these lesions show an increase in the number of micronuclei in their exfoliated oral mucosal cells and in circulating lymphocytes. Thus, the number of micronucleated oral mucosal cells can be used as a biomarker for predicting the clinical course of oral pre-cancers and early invasive cancer, and for assessing the potential of therapeutic agents.
One study evaluated the effects of alcoholic extracts of turmeric oil and turmeric oleoresin on the number of micronuclei in healthy participants and in patients with submucous fibrosis ( 27 ). None of the extracts had any effects on the number of micronuclei in lymphocytes from healthy participants. All three extracts, however, offered protection against benzo[a]pyrene-induced increase in micronuclei in patients circulating lymphocytes. In another set of experiments, patients with submucous fibrosis were given a daily oral dose of turmeric oil (600 mg) plus turmeric (3 g), turmeric oleoresin (600 mg) plus turmeric (3 g), or turmeric alone (3 g) for 3 months. Results indicated that all three treatment modalities decreased the number of micronucleated cells both in exfoliated oral mucosal cells and in circulating lymphocytes (Fig. 3b ). However, turmeric oleoresin was more effective in reducing the number of micronuclei in oral mucosal cells. These results suggest the potential of turmeric extract against micronuclei formation in patients with oral precancerous lesions.
Another phase I study evaluated the toxicology, pharmacokinetics, and biologically effective dose of curcumin in patients with resected urinary bladder cancer, arsenic-associated Bowen disease of the skin, uterine cervical intraepithelial neoplasm (CIN), oral leucoplakia, and intestinal metaplasia of the stomach ( 28 ). A total of 25 patients were enrolled in this study. Curcumin was given orally for 3 months, and biopsy of the lesion sites was done immediately before and 3 months after initiation of curcumin treatment. No treatment-related toxicity occurred with doses up to 8 g/day. At doses higher than 8 g/day, however, the bulky volume of the drug was unacceptable to the patients. The serum concentration of curcumin usually peaked at 1 to 2 h after curcumin intake and gradually declined within 12 h. However, urinary excretion of curcumin was undetectable. One of four patients with CIN and one of seven with oral leucoplakia developed frank malignancies in spite of curcumin treatment. In contrast, histologic improvement of precancerous lesions was seen in one of two patients with resected bladder cancer, two of seven patients of oral leucoplakia, one of six patients of intestinal metaplasia of the stomach, one of four patients with CIN, and two of six patients with Bowen disease ( 28 ). These data demonstrate the safety of curcumin at doses up to 8 g/day taken orally for 3 months. The study also suggested the chemopreventive potential of curcumin against cancerous lesions.
A randomized, double-blind, placebo-controlled trial was conducted in 100 patients with oral lichen planus to evaluate the efficacy of curcuminoids ( 29 ). The trial included two interim analyses, and the participants were randomly assigned to receive either placebo or curcuminoids at 2 g/day for 7 weeks. In addition, all participants received prednisone at 60 mg/day for the first week. The primary outcome was a change in symptoms from baseline, and secondary outcomes were changes in clinical signs and occurrence of any side effects. The results of the first interim analysis using data from 33 participants did not show any significant difference between the placebo and curcuminoid groups. Conditional power calculations suggested that the likelihood of the curcuminoid group having significantly better outcome than that of the placebo group if the trial were to be completed was less than 2%. Therefore, the study was ended before completion. However, curcuminoids were well-tolerated. For future studies of efficacy, the authors suggested the use of a larger sample size and a higher dose and/or longer duration of curcuminoids without an initial course of prednisone ( 29 ). Since the earlier studies had examined the effects of alcoholic extracts of turmeric, turmeric oil, and turmeric oleoresin in patients with submucous fibrosis ( 27 ) but the later study used curcumin ( 29 ), it remains unclear whether differences in the preparations accounted for the differences in results.
More recently, administration of a 1-g curcumin tablet (900 mg of curcumin, 80 mg of demethoxycurcumin, 20 mg of bisdemethoxycurcumin) for 1 week was associated with an increase in vitamins C and E levels and a decrease in MDA and 8-hydroxydeoxyguanosine (8-OHdG) contents in the serum and saliva of patients with precancerous lesions ( 30 ).
Head and Neck Squamous Cell Carcinoma
HNSCC is the sixth most common cancer worldwide, with approximately 600,000 cases diagnosed per year. HNSCC is a heterogeneous disease that includes oral, laryngeal, and pharyngeal malignancies, with about 40% of these arising in the oral cavity. Despite medical advancements, the 5-year survival rate for patients with HNSCC remains in the range of 40% to 50%. Studies over the past several years have indicated the role of NF–κB and inflammatory molecules such as IL-6, IL-8, and VEGF in the pathogenesis of this disease ( 86 ). Therefore, targeting these signaling molecules might prove useful against HNSCC. Whether curcumin can inhibit IκB kinase β (IKKβ) kinase activity, an enzyme involved in NF–κB activation that suppresses expression of inflammatory cytokines in patients with HNSCC, was investigated ( 31 ). A total of 39 patients (13 with dental caries, 21 with HNSCC, and 5 healthy volunteers) participated in this study. Saliva was collected before and 1 h after participants chewed two curcumin tablets for 5 min. Curcumin treatment led to a reduction in IKKβ kinase activity in the salivary cells of patients with HNSCC. Treatment of UM-SCC1 cell lines with curcumin as well as with post-curcumin salivary supernatant showed a reduction of IKKβ kinase activity. Significant reduction in IL-8 levels was seen in post-curcumin samples from patients with dental caries. Although IL-8 expression was reduced in 8 of 21 post-curcumin samples of patients with HNSCC, the data did not reach statistical significance. The authors of this study concluded that IKKβ kinase could be used as a biomarker for detecting the effect of curcumin in HNSCC ( 31 ).
Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) is a condition in which the intestines become inflamed. Although the etiology of IBD is not clearly known, it appears to be driven by inflammatory cytokines such as TNF-α. Two major types of IBD are ulcerative colitis and Crohn disease. Whereas ulcerative colitis is limited to the colon, Crohn disease can involve any part of the gastrointestinal tract from the mouth to the anus. Another mild-to-moderate form of ulcerative colitis is called ulcerative proctitis, which involves inflammation of the rectum. Patients with IBD have a significantly higher risk of developing colon cancer than the general population has. Generally, anti-inflammatory drugs, immunosuppressants, and TNF blockers are used to manage IBD. However, the high cost and adverse effects associated with these drugs encourages the use of alternative management options.
One open-label study evaluated the efficacy of curcumin in five patients with ulcerative proctitis and in five patients with Crohn disease ( 32 ). The patients with ulcerative proctitis were given 550 mg of curcumin twice daily for 1 month and then 550 mg three times daily for another month. In the patients with Crohn disease, curcumin was administered at a dose of 360 mg three times a day for 1 month and then 360 mg four times a day for another 2 months. Significant decrease in symptoms as well as in inflammatory indices (erythrocyte sedimentation rate and CRP) were observed in all patients with proctitis. Only four of the five patients with Crohn disease, however, completed the study. There was a mean reduction of 55 points in the Crohn disease activity index, and reductions in erythrocyte sedimentation rate and CRP were observed in these patients. Although this study suggests the efficacy of curcumin against IBD, large double-blind, placebo-controlled studies are required for confirmation.
Another study evaluated the efficacy of curcumin as maintenance therapy in 89 patients with quiescent ulcerative colitis ( 33 ). For this randomized, double-blind, multicenter trial, 45 patients received curcumin, 1 g after breakfast and 1 g after the evening meal, plus sulfasalazine or mesalamine, and 44 patients received placebo plus sulfasalazine or mesalamine for 6 months. The relapse rates were 4.65% in the curcumin-treated group and 20.51% in the placebo group (Fig. 4a ).
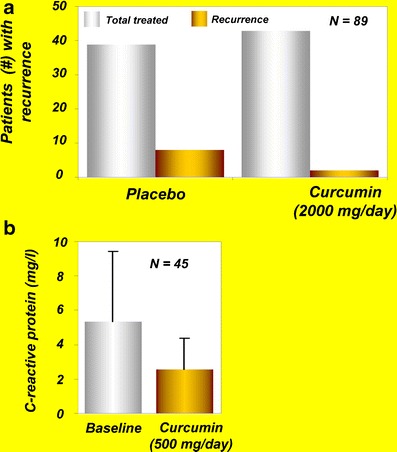
a Effects of curcumin on recurrence of disease in patients with ulcerative colitis [reprinted from Clinical Gastroenterology and Hepatology, vol 4, Hanai et al. , Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial, pages 1502–1506, copyright (2006), with permission from Elsevier ( 33 )]. b Levels of C-reactive protein in patients with active rheumatoid arthritis at baseline and after curcumin treatment [reprinted with permission from Chandran and Goel, (2012), Phytotherapy Research, John Wiley and Sons ( 39 )]
In another recent study, ingestion of oral curcumin at 500 mg/day along with prednisone was associated with clinical and endoscopic remission in a 60-year-old woman with a 17-year history of left-sided ulcerative colitis and enteropathic arthropathy ( 34 ). The patient had been examined for persistently active colitis in December 2009. Both a clinical and endoscopic evaluation confirmed the diagnosis. Previously, multiple mesalamine preparations, sulfasalazine, and steroid enemas had not been effective, and the patient had required multiple courses of steroids for disease exacerbation. She refused azathioprine/6-mercaptopurine and anti-TNF treatment because of possible adverse effects. In addition to 40 mg of prednisone, 500 mg of curcumin per day was given to the patient. After receiving curcumin and prednisone treatment for 1 year, the patient’s bowel movements had gone to two per day without blood, she was no longer taking steroids, and she was feeling well. She remained in clinical remission at further clinical evaluations in April, July, and December 2010. A colonoscopy performed in September 2010 showed no ulceration and biopsies consistent with chronic inactive ulcerative colitis ( 34 ). Thus, based on this case study, curcumin represents a viable treatment alternative or adjunctive therapy in the management of chronic ulcerative colitis.
A recent study assessed the effect of curcumin on the levels of p38 mitogen-activated protein kinase (p38 MAPK), IL-1β, IL-10, and matrix metalloproteinase-3 (MMP-3) in the gut of children and adults with IBD ( 35 ). Colonic mucosal biopsies and colonic myofibroblasts from children and adults with active IBD were cultured ex vivo with curcumin. Results indicated suppression in p38 MAPK activation, reduction in IL-1β, and enhancement in IL-10 levels in curcumin-treated mucosal biopsies. Furthermore, dose-dependent suppression of MMP-3 in colonic myofibroblasts was observed after curcumin treatment ( 35 ).
Irritable Bowel Syndrome
Irritable bowel syndrome (IrBS) is a chronic problem of the large intestine. The most common symptoms of IrBS are cramping, abdominal pain, bloating, gas, diarrhea, and constipation. The causes of IrBS are unclear, and there is no commonly accepted cure. A partially blinded, randomized, two-dose, pilot study assessed the effects of turmeric extract on IrBS symptoms in healthy adults ( 36 ). Turmeric was given to the volunteers in tablet form: 102 patients were given one tablet containing 72 mg of standardized turmeric extract, and 105 patients were given two tablets a day, both for 8 weeks. The prevalence of IrBS was reduced by 53% and 60% in the one-tablet and two-tablet groups, respectively, and was associated with a marked decrease in IrBS symptoms ( 36 ). Although these results suggest that turmeric may help reduce IrBS symptoms, placebo-controlled trials are needed to confirm these findings. Another study conducted with eight healthy participants reported that turmeric has the potential to increase bowel motility and to activate hydrogen-producing bacterial flora in the colon ( 37 ).
Arthritis is a chronic disease that results from the inflammation of one or more joints. It usually results from dysregulation of pro-inflammatory cytokines ( e.g ., TNF, IL-1β) and pro-inflammatory enzymes that mediate the production of prostaglandins ( e.g ., COX-2) and leukotrienes ( e.g ., lipoxygenase), together with the expression of adhesion molecules and matrix metalloproteinases. Although more than 100 different kinds of arthritis have been reported, the three most common forms are osteoarthritis, rheumatoid arthritis, and gout. Typically, a combination of exercise, modifications in lifestyle factors, and NSAIDs are used for the treatment of osteoarthritis. The use of NSAIDs, however, is associated with numerous adverse effects.
The potential of curcumin against arthritis was first reported in 1980 in a short-term, double-blind, crossover study involving 18 young patients with rheumatoid arthritis ( 38 ). In this study, curcumin’s efficacy was compared with that of the prescription drug phenylbutazone. Patients were randomly assigned to receive either curcumin (1.2 g/day) or phenylbutazone (0.3 g/day) for 2 weeks. Curcumin was well-tolerated, had no adverse effects, and exerted an anti-rheumatic activity identical to that of phenylbutazone as shown by improvement in joint swelling, morning stiffness, and walking time. However, one of the major drawbacks of this study was the lack of a control or placebo group ( 38 ). Further well-controlled studies are therefore required to examine the long-term effects of curcumin against rheumatoid arthritis. In another recent study, curcumin alone (0.5 g) and in combination with diclofenac sodium (0.05 g) was found to be safe and effective in 45 patients with rheumatoid arthritis ( 39 ). Furthermore, the level of CRP was suppressed in these patients after curcumin administration (Fig. 4b ).
Another study in 50 patients with osteoarthritis evaluated the efficacy of Meriva at a dose that corresponded to 200 mg of curcumin per day ( 40 ). The signs and symptoms of osteoarthritis were evaluated with use of WOMAC scores, an indicator of pain level. The mobility was assessed by walking performance (treadmill), and inflammatory status was assessed by measuring the levels of CRP. After 3 months of treatment, the global WOMAC score was decreased by 58%; walking distance was increased from 76 m to 332 m, and CRP levels were significantly decreased. In comparison, only modest improvement in these measurements was observed in the control group. Overall, these results suggested the efficacy of Meriva in the management of osteoarthritis ( 40 ). In a subsequent study, this group investigated the long-term efficacy and safety of Meriva in a longer (8-month) study involving 100 patients with osteoarthritis ( 41 ). The patients were divided into the control group (50 patients) and the curcumin group (50 patients), in which patients received 1 g/day of Meriva for 8 months. The WOMAC score was decreased by more than 50%, whereas treadmill walking performance was increased almost threefold compared with the control. Serum inflammatory biomarkers such as IL-1β, IL-6, soluble CD40 ligand, soluble vascular cell adhesion molecule-1, and erythrocyte sedimentation rate were also significantly decreased in the treatment group. In addition, remarkable decreases in gastrointestinal complications, distal edema, and the use of NSAIDs/painkillers by the patients were also noted after Meriva treatment. The need for hospital admissions, consultations, and tests by the patients was also decreased after Meriva treatment. The authors of this study concluded that Meriva is worth considering for the long-term complementary management of osteoarthritis ( 41 ).
Uveitis is an inflammation of the uvea, the middle layer of the eye. Uveitis is a major cause of visual impairment and has been estimated to account for 10% to 15% of all cases of total blindness in the United States. Depending on the anatomical localization and visible signs of the disease, uveitis can be classified into anterior, posterior, pan, and intermediate. The course of the disease can be acute, chronic (>3-month duration), and recurrent. Corticosteroids are normally used for treatment of uveitis. However, the adverse effects associated with these drugs limit their use.
One study evaluated the efficacy of curcumin against chronic anterior uveitis ( 42 ). Curcumin was administered orally to patients with chronic anterior uveitis at a dose of 375 mg three times a day for 12 weeks. Of 53 patients enrolled, 32 completed the 12-week study and were divided into two groups. One group of 18 patients received curcumin alone, whereas the other group of 14 patients, who had a strong reaction to tuberculin purified protein derivative, also received anti-tubercular treatment. After 2 weeks of treatment, both groups showed significant improvement in the disease. Whereas all patients who received curcumin alone exhibited improvement, the group receiving anti-tubercular therapy along with curcumin had a response rate of 86%. Furthermore, follow-up of all patients for the next 3 years found recurrence rates of 55% for the first group and 36% for the second group. However, 22% of patients in the first group and 21% of patients in the second group lost their vision in the follow-up period due to various complications in the eyes. The efficacy of curcumin on recurrences after treatment was comparable to that of corticosteroid therapy. Furthermore, lack of any adverse effects with curcumin was an advantage over corticosteroid therapy ( 42 ). A double-blind, multicenter clinical trial with curcumin against chronic anterior uveitis is highly desirable to further validate the results of this study.
One nonplacebo-controlled study evaluated the efficacy of Meriva against recurrent anterior uveitis ( 43 ). The study group consisted of 106 patients divided into three main groups of different uveitis origin: group 1 (autoimmune uveitis, 56 patients), group 2 (herpetic uveitis, 28 patients), and group 3 (various etiologies of uveitis, 22 patients). All patients were given Norflo containing 600 mg of Meriva twice daily during the follow-up period (about 12–18 months). The primary end point was relapse frequency in all treated patients, before and after Meriva treatment, followed by the number of relapses in the three etiological groups. The secondary end points were relapse severity and overall quality of life. A total of 106 and 19 patients, respectively, had relapses before and after treatment with Norflo. Furthermore, the total number of relapses was reduced from 275 to 36 after the 1-year treatment with Norflo. Meriva was well-tolerated and reduced eye discomfort after a few weeks of treatment in more than 80% of patients. Thus, the study demonstrated the therapeutic role of curcumin and its efficacy against recurrent anterior uveitis ( 43 ).
Postoperative Inflammation
In a study of curcumin’s anti-inflammatory properties, Satoskar et al. ( 44 ) evaluated the effects of this polyphenol on spermatic cord edema and tenderness in 46 men (15–68 years old) who had just undergone surgical repair of an inguinal hernia and/or hydrocele. After surgery, patients were randomly assigned to receive curcumin (400 mg), placebo (250 mg lactose powder), or phenylbutazone (100 mg) three times a day for 6 days. Spermatic cord edema, spermatic cord tenderness, operative site pain, and operative site tenderness reflected by intensity score (TIS) were measured. TIS on day 6 decreased by 84.2% in the curcumin group, by 61.8% in placebo group, and by 86% in phenylbutazone group. Although TIS values for the curcumin and phenylbutazone groups were similar on day 6, curcumin proved to be superior by reducing all four measures of inflammation ( 44 ).
Peptic Ulcer
Peptic ulcers are the most common ulcer of the gastrointestinal tract and can be extremely painful. These ulcers are usually open sores that develop on the inner lining of the esophagus, stomach, and the upper portion of the small intestine. If the peptic ulcer is located in the stomach, it is called a gastric ulcer. According to one estimate, 5% to 10% of adults globally are affected by peptic ulcers at least once in their lifetime. The preferred medications for peptic ulcers include proton pump inhibitors, histamine receptor blockers, and antibiotics to kill a Helicobacter pylori infection. A randomized controlled clinical trial from Thailand compared the efficacy of turmeric and liquid antacid (containing 333 g of aluminum hydroxide and 33.3 g of magnesium hydroxide per 1,000 ml) against benign gastric ulcers ( 45 ). Of the 60 patients who participated in the study, 30 received turmeric (250 mg, four times per day), and the other 30 received antacid (30 ml, four times per day). The treatment was continued for 6 to 12 weeks. Although both antacid and turmeric improved gastric ulcers in patients, the former was better in reducing the ulcers ( 45 ).
A phase II clinical trial from Thailand evaluated the safety and efficacy of curcumin in patients with peptic ulcers ( 46 ). Forty-five patients (24 men and 21 women, aged 16–60 years) were included in the study. Twenty-five patients (18 men and 7 women) underwent endoscopy, and their ulcers were found in the duodenal bulb and gastric (angulus) region. The remaining 20 patients did not have ulcers but appeared to have erosions, gastritis, and dyspepsia. Two capsules (300 mg each) of turmeric were given orally five times daily over a period of 4 weeks. Results after 4 weeks of treatment showed that ulcers were absent in 12 patients; after 8 weeks of treatment, ulcers were absent in 18 patients; and after 12 weeks of treatment, ulcers were absent in 19 patients. The remaining patients had symptomatic relief after turmeric treatment ( 46 ).
H . pylori Infection
H . pylori is one of the most widespread infectious agents and is the common cause of peptic ulcers. The bacterium is also involved in the pathogenesis of several other diseases, such as mucosa-associated lymphoid tissue lymphoma, gastric adenocarcinoma, iron deficiency anemia, skin disease, and rheumatologic conditions ( 87 ). The most commonly used treatment regimens for H. pylori infection include the use of proton pump inhibitors and antibiotics. However, these medications are associated with adverse effects. One study investigated the effectiveness of 7-day non-antibiotic therapy (including curcumin, lactoferrin, N -acetylcysteine, and pantoprazole) for eradication of H. pylori infection and reduction of gastric inflammation ( 47 ). Twenty-five H. pylori -positive patients with functional dyspepsia were enrolled in the study, and the outcomes evaluated were H. pylori eradication, gastric inflammation, and relief of symptoms. Patients were treated twice a day for 7 days with curcumin (30 mg), bovine lactoferrin (100 mg), N -acetylcysteine (600 mg), and pantoprazole (20 mg). H. pylori status and upper gastrointestinal symptoms were assessed by 13 C-urea breath test and an intensity scale for upper gastrointestinal symptoms (absent, mild, moderate, and severe), as well as a blood test for serum pepsinogens (sPGI, sPGII), gastrin-17 (G-17), and anti- H. pylori IgG (IgG-Hp) at baseline and after 2 months. Results indicated that 3 (12%) of 25 patients were cured of H. pylori infection. Significant decreases in the overall severity of symptoms and in sPGII and sPGI levels were observed after 2 months of the treatment. However, IgG and G-17 values did not significantly decrease after 2 months. The authors of this study concluded that the therapy is not effective for H. pylori eradication. However, significant improvement in dyspeptic symptoms and a reduction of serologic signs of gastric inflammation were observed after 2 months ( 47 ). Additional studies with larger cohorts of participants are necessary to confirm the potential of curcumin in the management of H. pylori infection.
Another study investigated the effect of curcumin on the production of IL-8, IL-1β, TNF-α, and COX-2 in gastric mucosa from 36 H. pylori- infected gastritis patients ( 48 ). The patients were randomly assigned to receive either a 1-week course of OAM-based triple regimen (20 mg of omeprazole, 1 g of amoxicillin, and 800 mg of metronidazole, each given orally twice a day) or a 4-week course of turmeric tablets (700 mg containing 40 mg of curcumin, three times a day). Gastric biopsy samples were collected before and after treatment and were examined for the level of inflammatory cytokines. The eradication rate of H. pylori was significantly higher for patients who received OAM treatment than it was for patients who received curcumin. The levels of IL-8 mRNA expression in the OAM group significantly decreased after treatment, but no changes of other cytokines were found. However, decrease in cytokine production was not found in the curcumin group. The study concluded that curcumin alone may have a limited anti-bactericidal effect on H. pylori and on the production of inflammatory cytokines ( 48 ).
Idiopathic Orbital Inflammatory Pseudotumor
Idiopathic orbital inflammatory pseudotumor (IOIP) is a chronic neoplasm-like inflammatory reaction, usually affecting the orbital tissues of both eyes and orbit. Originally characterized in 1905 by Birch-Hirschfeld, the disease constitutes the third most common ophthalmic disorder after Grave’s disease and lymphoproliferative disorders ( 88 ). Oral corticosteroids, radiotherapy, or anti-metabolites such as cyclophosphamide are normally used for the treatment of the disease; however, 25% to 50% of patients do not respond ( 89 ). The clinical efficacy of curcumin in the treatment of IOIP was investigated in a study of eight patients ( 49 ), in which curcumin was administered orally at a dose of 375 mg, three times a day, for a period of 6 to 22 months. The patients were followed up for 2 years at 3-month intervals. Five patients completed the study; of these, four recovered completely, and in one patient, the swelling regressed completely but some limitation of movement persisted. Furthermore, the disease did not recur in any of the patients, and curcumin was not associated with any adverse effects. On the basis of these observations, the authors of this study concluded that curcumin could be used as a safe and effective drug in the treatment of IOIP ( 49 ). However, well-controlled multicenter clinical trials are needed to confirm the efficacy of curcumin against IOIP.
Vitiligo is a skin disorder in which the cells producing pigment (color) in the skin (melanocytes) are destroyed, resulting in white patches that appear on the skin on different parts of the body. Although what causes damage to melanocytes remains unclear, oxidative stress has been implicated in the pathogenesis of the disease ( 90 ). Narrowband UVB (NB-UVB) that uses the portion of the UVB spectrum from 311 to 312 nm is now considered the gold standard treatment for vitiligo ( 91 ). Because of its anti-oxidant property, curcumin seems to be a therapeutic option for the treatment of vitiligo. One study investigated whether the combination of NB-UVB and tetrahydrocurcuminoid cream could result in synergistic therapeutic effects against vitiligo ( 50 ). Ten patients with focal or generalized vitiligo were enrolled in the study. Two similar lesions were treated with either NB-UVB plus topical tetrahydrocurcuminoid cream or with UVB alone. The UVB treatments were given twice a week for 12 weeks. Results indicated a statistically significant repigmentation in both treatment groups compared with baseline on completion of the study. Furthermore, the overall degree of repigmentation was slightly better in the combination group at 8 and 12 weeks, and the tetrahydrocurcuminoid was well-tolerated ( 50 ).
Psoriasis is a chronic inflammatory skin disease characterized by thick, red, scaly lesions that may appear on any part of the body. The disease exists in five different forms—plaque, guttate, inverse, pustular, and erythrodermic—of which plaque psoriasis is most common. The disease affects approximately 2% of the population worldwide and is associated with increased cardiovascular risk ( 92 ). The currently available treatment for psoriasis is time-consuming (UVB or psoralen plus UVA therapy) and has the potential for organ toxicity (methotrexate, acitretin, cyclosporine).
Elevations of activity in PhK, a serine/threonine-specific protein kinase, have been correlated with pathogenesis of psoriasis. Therefore, agents with potential to inhibit PhK activity can be useful for the treatment of psoriasis. One of the early studies from our own laboratory indicated that curcumin is a noncompetitive inhibitor of PhK, with a Ki of 75 μM ( 93 ). A different study investigated whether the anti-psoriatic activity of curcumin in patients is due to suppression of PhK activity ( 51 ). In this study, PhK activity was assayed in four groups of ten participants each: (1) active untreated psoriasis; (2) resolving psoriasis treated by calcipotriol, a vitamin D 3 analogue and an indirect inhibitor of PhK; (3) curcumin treatment (1% in the gel); and (4) normal non-psoriatic participants. The PhK activity was highest in active untreated psoriasis and progressively lower in the calcipotriol-treated group, in the curcumin-treated group, and in non-psoriatic participants (Fig. 5a ). The decrease in PhK activity in curcumin- and calcipotriol-treated psoriasis was associated with a decrease in keratinocyte transferrin receptor expression and with decrease in the severity of parakeratosis and the density of epidermal CD8+ T cells. The authors of this study concluded that drug-induced suppression of PhK activity is associated with resolution of psoriatic activity and that the anti-psoriatic activity of curcumin may be achieved through modulation of PhK activity ( 51 ). However, further well-controlled clinical trials are required to confirm these observations.
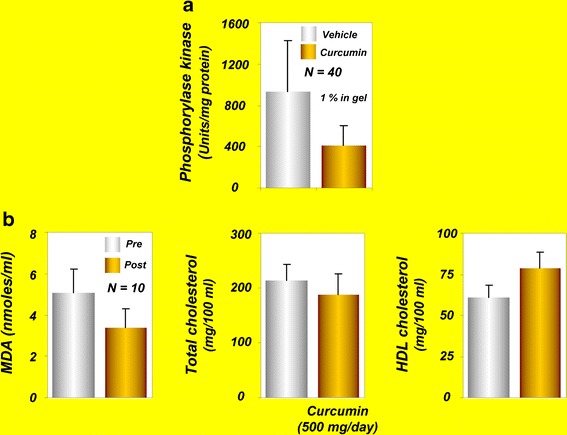
a Phosphorylase kinase values in curcumin- and vehicle-treated groups [reprinted with permission from Heng et al. , (2000), British Journal of Dermatology , John Wiley and Sons ( 51 )]. b Effects of curcumin on serum MDA and lipoproteins in human volunteers [reprinted from Soni and Kuttan, 1992, with permission of Executive Editor, Indian Journal of Physiology and Pharmacology ( 57 )]. HDL , high density lipoprotein; MDA , malondialdehyde
A phase II, open-label, Simon’s two-stage clinical trial sought to determine the safety and efficacy of oral curcumin in patients with moderate to severe psoriasis ( 52 ). Twelve patients with chronic plaque psoriasis were enrolled in the study and were given 4.5-g curcumin capsules every day for 12 weeks, followed by a 4-week observation period. Curcumin was well-tolerated, and all participants completed the study. The response rate was low, however, possibly caused by a placebo effect or the natural history of psoriasis. However, two patients who responded to the treatment showed 83% to 88% improvement at 12 weeks of treatment. Small sample size and the lack of a control (placebo) group were the limitations of the study ( 52 ). Therefore, large placebo-controlled studies are required before recommending oral curcumin for psoriasis.
Dejerine-Sottas Disease
Dejerine-Sottas disease is a severe degenerative form of Charcot-Marie-Tooth disease, a neurological disorder. The disease is characterized by generalized weakness sometimes progressing to severe disability, loss of sensation, curvature of the spine, and sometimes mild hearing loss. The disease is caused by defects in genes of axons and myelin such as myelin P0 (MPZ), peripheral myelin protein 22 (PMP22), PRX, and EGR2. One study assessed the safety of oral curcumin in a 15-year-old Caucasian girl with Déjérine-Sottasdisease ( 53 ). The patient received 1.5 g of oral curcumin daily for the first 4 months and 2.5 g/day thereafter, to complete a 12-month trial. After 12 months, the patient experienced no adverse events and reported good compliance. Knee flexion and foot strength increased slightly, but hand and elbow strength decreased. Pulmonary function, hand function, and measures of upper/lower extremity disability were stable or reduced. The neurophysiologic findings of the patient were unchanged. Parent-reported quality of life improved for most domains, especially self-esteem, during the 12 months of treatment. Overall, these results suggest the safety and efficacy of curcumin against Déjérine-Sottas disease ( 53 ). A well-controlled, randomized, large clinical trial is needed to confirm the efficacy of curcumin against this disease.
Alzheimer’s Disease
Alzheimer’s disease is a progressive neurodegenerative disorder, usually affecting people older than age 65 years. The pathogenesis of Alzheimer’s disease involves aggregation of Aβ (especially Aβ 1–42 ) into fibrils, formation of amyloid plaques, and deposition of these plaques into the brain. These plaques are believed to cause the loss of cholinergic neurons in the basal forebrain of patients with Alzheimer’s disease ( 94 ). The currently available treatments for this disease have numerous adverse effects, thus underscoring the need for alternative approaches. A phase II, randomized, double-blind, placebo-controlled study in the United States was designed to evaluate the safety and tolerability of curcumin in patients with mild to moderate Alzheimer’s disease ( 54 ). A total of 33 patients who were enrolled in the study were randomly assigned to a placebo group, low-dose curcumin group (2 g/day), or high-dose curcumin group (4 g/day). After 24 weeks, the patients who were receiving curcumin continued the treatment at their assigned dose, whereas those who were receiving the placebo were given one of the two doses of curcumin. The study examined the safety, tolerability, pharmacokinetics, and efficacy of curcumin in patients with Alzheimer’s disease, as well as the effects of curcumin on biomarkers associated with the pathology of this disease. Although the study has been completed, the observations have yet to be published ( 54 ).
Baum et al. ( 55 ) conducted a randomized, double-blind, placebo-controlled study in 34 patients with Alzheimer’s disease. The study participants were randomly assigned to receive curcumin at two different doses (1 or 4 g) or placebo (4 g). The Mini-Mental State Examination (MMSE) score that assesses mental status was not improved after curcumin treatment. Similarly, the level of serum Aβ40 was not affected by curcumin treatment. However, curcumin administration was associated with an increase in vitamin E level, and curcumin did not cause any adverse effects. These authors concluded that the anti-oxidant activity of curcuminoids might decrease the need for anti-oxidant vitamin E ( 55 ). These observations support the opening of a clinical trial of curcumin against Alzheimer’s disease using large numbers of patients.
Acute Coronary Syndrome
Acute coronary syndrome (ACS) refers to a situation in which the blood supply to the myocardium is cut off. ACS encompasses three clinical conditions involving the coronary arteries: ST elevation myocardial infarction (STEMI), non-ST elevation MI, and unstable angina. Dyslipidemia and hyperglycemia are characteristic features of patients with ACS ( 95 ). A randomized, double-blind, controlled trial from Jakarta evaluated the effects of curcumin on total cholesterol, LDL cholesterol, HDL cholesterol, and triglyceride levels in patients with ACS ( 56 ). A total of 70 patients were assigned to four different groups: placebo, low-dose (45 mg/day), moderate-dose (90 mg/day), and high-dose (180 mg/day) curcumin. The curcumin was administered orally to the patients for 2 months. Low-dose curcumin was highly effective, compared with high-dose curcumin, in reducing total cholesterol and LDL cholesterol in patients. Conversely, low-dose curcumin increased HDL cholesterol to a greater extent than did the high-dose. The increase in triglyceride content by curcumin was greatest at the moderate dose, however. These studies suggest the beneficial effects of curcumin in improving lipid profiles in patients with ACS ( 56 ). However, improving the lipid profile does not necessarily mean that curcumin is effective against ACS. Further studies are required to demonstrate whether curcumin can suppress ACS in patients.
Atherosclerosis
Atherosclerosis is a condition in which fatty materials such as cholesterol accumulate and thickens the artery wall ( 96 ). This is a chronic disease that normally remains asymptomatic for decades. One study evaluated the effects of curcumin in reducing the serum levels of cholesterol and lipid peroxides in ten healthy human volunteers ( 57 ). Curcumin (at 0.5 g/day) administered to the volunteers for 7 days reduced serum lipid peroxides by 33% and total serum cholesterol levels by 11.63%, and increased HDL cholesterol by 29% (Fig. 5b ). Because of these properties, curcumin was suggested to act as a chemopreventive agent against atherosclerosis.
Diabetes mellitus (DM) is a chronic metabolic disease in which a person has high concentrations of blood sugar. The high blood sugar in turn produces symptoms of polyuria, polydipsia, and polyphagia. Three main types of diabetes are type 1, type 2, and gestational diabetes. Type 1 result from the body’s failure to produce insulin, whereas in type 2 diabetes (T2DM) the body fails to use insulin properly. Extensive research over the past several years has indicated that pro-inflammatory cytokines and oxidative stress play a role in the pathogenesis of T2DM ( 97 ). Because of its anti-inflammatory property, curcumin represents a promising therapeutic option for T2DM. Curcumin’s ability to decrease blood sugar levels in human patients was first reported in 1972 ( 58 ). A male patient who had diabetes for 16 years ingested 5 g of turmeric powder over a period, after which his fasting blood sugar decreased from 140 to 70 mg/dl. Ingestion of turmeric or curcumin along with insulin synergistically reduced the blood sugar level. Furthermore, when the insulin dosage was decreased to the minimum, the anti-diabetic effect of turmeric was persistent. Interestingly, when the ingestion of curcumin and turmeric was discontinued for a week, random blood sugar levels increased to 140 mg/dl. Therefore, ingestion of a daily 5-g dose of turmeric was resumed, which promptly reduced the fasting blood sugar level to 110 mg/dl. Blood urea in this patient after 3 months of turmeric therapy was 20 to 22, and the patient’s electrocardiogram was normal. Turmeric therapy was not associated with any palpable adverse effects; rather, the beneficial effects of turmeric as a good appetite stimulant and effective laxative were observed ( 58 ).
Usharani et al. ( 59 ) evaluated the potential of a standardized preparation of curcuminoids (NCB-02) against various oxidative stress and inflammatory markers in patients with T2DM. Seventy-two patients with T2DM were randomly assigned to receive NCB-02 (300 mg of curcumin, twice a day), atorvastatin (10 mg, once a day), or placebo for 8 weeks. Of the 72 patients, 67 completed the study. Curcumin treatment significantly improved endothelial function and reduced oxidative stress (MDA) and inflammatory markers (IL-6, TNFα, endothelin-1) in these patients. Larger, randomized clinical trials should further confirm the observations of this proof-of-concept study.
Another study examined the effects of C. longa on postprandial plasma glucose and insulin levels and the glycemic index in healthy participants ( 60 ). Fourteen healthy participants were assessed in a crossover trial. The study found that ingestion of C. longa increased postprandial serum insulin levels but had no effect on plasma glucose levels or the glycemic index in these healthy participants. The study concluded that C. longa might have an effect on insulin secretion ( 60 ).
More recently, a randomized, double-blind, placebo-controlled clinical trial assessed the efficacy of curcumin in delaying development of T2DM in the prediabetes population ( 61 ). A total of 240 participants were randomly assigned to receive either curcumin (1.5 g/day) or placebo capsules, and changes in β cell functions (homeostasis model assessment [HOMA]-β, C-peptide, and proinsulin/insulin), insulin resistance (HOMA-IR), and anti-inflammatory cytokine (adiponectin) levels were monitored at the baseline and at 3, 6, and 9 months of treatment. After 9 months of treatment, 16.4% of participants in the placebo group were diagnosed with T2DM, whereas none were diagnosed with T2DM in the curcumin-treated group (Fig. 6a ). In addition, the participants of curcumin-treated group showed a better overall function of β cells, with higher HOMA-β and lower C-peptide levels. The curcumin-treated participants also exhibited a lower level of HOMA-IR and higher adiponectin when compared with the placebo group. The authors of this study concluded that the curcumin may be beneficial in a prediabetes population ( 61 ).
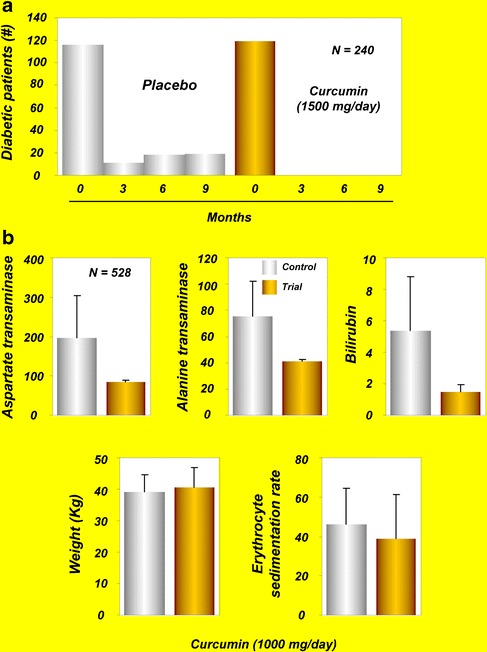
a Number of newly diagnosed diabetic subjects after treatment with curcumin [copyright 2012 American Diabetes Association From Diabetes Care (R) , vol. 35, 2012, reprinted by permission of the American Diabetes Association ( 61 )]. b Effects of C . longa and Tinospora formulation on liver aspartate transaminase, alanine transaminase, bilirubin, and body weight and erythrocyte sedimentation rate in tuberculosis patients [reprinted with permission from Adhvaryu et al. , (2008), World Journal of Gastroenterology ( 71 )]
Type 2 Diabetic Nephropathy
End-stage renal disease due to type 2 diabetic nephropathy is a very common condition that is associated with high worldwide levels of mortality and morbidity. Both proteinuria and TGF-β may contribute to the development of end-stage renal disease in patients with diabetic nephropathy. One study investigated the effects of turmeric on serum and urinary TGF-β, IL-8, and TNF-α, as well as proteinuria, in patients with overt type 2 diabetic nephropathy ( 62 ). The study consisted of 40 patients with overt type 2 diabetic nephropathy who were randomly assigned to either the trial group ( n = 20) or the control group ( n = 20). Each patient in the trial group received one capsule (containing 500 mg of turmeric, three times a day) with each meal for 2 months; the control group received placebo capsules containing starch for the same 2 months. Serum concentrations of TGF-β and IL-8 and urinary protein excretion and IL-8 were significantly decreased after turmeric supplementation in comparison with the pre-supplementation values. No adverse effects related to turmeric supplementation were observed during the trial. The authors of this study concluded that short-term turmeric supplementation can attenuate proteinuria, TGF-β, and IL-8 in patients with overt type 2 diabetic nephropathy and can be administered as a safe adjuvant therapy for these patients ( 62 ). However, long-term trials with larger numbers of patients are needed to confirm whether turmeric’s effects on renal function are transient or long-lasting.
Diabetic Microangiopathy
Microangiopathy is a disease of the small blood vessels (capillaries), in which the capillary walls become so thick and weak that they bleed, leak protein, and slow the flow of blood. Hyperglycemia in patients with poorly controlled diabetes induces biochemical and molecular changes in microvascular cells that lead to retinal, renal, and neural complications and ultimately extend to other complications, including advanced periodontal disease. The treatment of diabetic microangiopathy is based on control of glycemia, lipemia, and blood pressure using glitazones, angiotensin II receptor antagonists, and statins ( 98 ).
One study evaluated the potential of Meriva in improving diabetic microangiopathy ( 63 ). In these patients, the disease was associated with microcirculatory alteration that was managed without insulin for at least 5 years. All patients were treated with what could be considered the best treatment protocol for the disease. In the treatment group, Meriva (1 g/day) was added as a supplement to the standard treatment for 4 weeks and was well-tolerated, with no dropouts reported, and all participants in the treatment and control group completed the study. In the treatment group, at 4 weeks, microcirculatory and clinical evaluations indicated a decrease in skin flux at the surface of the foot, an indicator of an improvement in microangiopathy. Also, a significant decrease in the edema score and a corresponding improvement in the venoarteriolar response were observed. An increase in PO 2 , possibly due to better oxygen diffusion into the skin and decreased edema, was observed. These features were observed in all participants using Meriva, whereas no clinical or microcirculatory effects were observed in the control group ( 63 ).
These results suggest the usefulness of Meriva for the management of diabetic microangiopathy and open a window of opportunities for the evaluation of curcumin’s efficacy in more prolonged and larger studies.
Lupus Nephritis
Lupus nephritis is an autoimmune disease characterized by polyclonal B cell hyperactivity and defective T cell function. The disease is responsive to immunosuppressive and steroid therapy, but sometimes the disease relapses. The effect of oral turmeric supplementation on 24 patients with relapsing or refractory biopsy-proven lupus nephritis was investigated in a randomized and placebo-controlled study ( 64 ). The patients in the trial group were given one capsule containing 500 mg of turmeric with each meal for 3 months. The patients in the control group received capsules containing starch that were identical in color and size to the turmeric capsules. A significant decrease in proteinuria was observed in the trial group compared with the control group. Turmeric supplementation significantly lowered systolic blood pressure and hematuria in patients. It was concluded that short-term turmeric supplementation can decrease proteinuria, hematuria, and systolic blood pressure in patients with relapsing or refractory lupus nephritis and can be used as a safe adjuvant therapy for such patients ( 64 ). Long-term clinical trials with larger numbers of patients are required to further clarify these effects of turmeric.
Renal Transplantation
Renal transplantation is the transplantation of a kidney into a patient with end-stage renal disease. Kidneys for transplantation come from a living donor or a deceased (cadaver) donor. Delayed graft function is a common occurrence in cadaveric renal transplantation and has been linked to increased rates of acute rejection and reduced graft survival ( 99 ). One study examined the effects of curcumin and quercetin on early graft function in 43 dialysis-dependent cadaveric kidney recipients ( 65 ). Curcumin (480 mg) and quercetin (20 mg) were given in a single capsule to the patients for 1 month after surgery. The patients were randomly assigned to three groups: control (placebo), low-dose (one capsule, one placebo), and high-dose (two capsules). Delayed graft function was defined as dialysis need in the first week, slow graft function as failure of creatinine levels to decrease within the first 48 hand/or creatinine >2.5 mg/dl by day 10, and early function as the rest of the patients. There were four withdrawals: one by patient choice and three for urine leak. Results indicated that two patients in the control group exhibited delayed graft function, which was completely absent in either of the treatment groups. Incidences of early function were 43% in the control group, 71% in the low-dose group, and 93% in the high-dose group. Serum creatinine was significantly lower at 2 days (control, 7.6 ± 2.1; low, 5.4 ± 0.6; high, 3.96 ± 0.35) and at 30 days (control, 1.82 ± 0.16; low, 1.65 ± 0.09; high, 1.33 ± 0.1). The incidences of acute rejection within 6 months were14.3% in the control and low-dose groups and 0% in the high-dose group. Tremor was detected in 13% of the high-dose group and in 46% of the control and low-dose groups. Furthermore, urinary HO-1 activity was increased in a dose-dependent manner in the treatment groups. The authors of this study concluded that curcumin and quercetin can improve early outcomes in cadaveric renal transplantation, possibly through induction of HO-1 ( 65 ).
Acquired Immunodeficiency Syndrome
Acquired immunodeficiency syndrome (AIDS) is caused by the human immunodeficiency virus (HIV), which interferes with and weakens the immune system. The virus primarily infects vital components of the human immune system, such as CD4+ T cells, macrophages, and dendritic cells. The virus directly and indirectly destroys CD4+ T cells. Current treatment for HIV infection consists of highly active anti-retroviral therapy. A clinical trial from New England examined the effectiveness of curcumin as an anti-viral agent in 40 AIDS patients ( 66 ). Two participants dropped out due to adverse events unrelated to the curcumin study. Of the remaining 38 patients, 23 were randomly assigned to a high-dose group (2.5 g/d) and 15 to a low-dose group. The treatment was continued for 8 weeks. No evidence of curcumin-associated reduction in viral load was observed. CD4 cells showed a slight increase in the high-dose group and a consistent decrease in the low-dose group. However, none of the results was statistically significant. Despite the lack of apparent anti-viral or CD4 effects, most participants liked taking curcumin because they felt better ( 66 ). It is likely that curcumin could provide benefits in unknown ways.
β-Thalassemia
β-Thalassemia is an inherited blood disorder in which the body makes an abnormal form of β-chains of hemoglobin (Hb). The disorder results in excessive destruction of RBCs, which leads to anemia. In Southeast Asians, HbE, a common Hb variant, is normally associated with the β-thalassemia phenotype. Disturbances in oxidative stress and in the anti-oxidant defense system are commonly reported in patients with β-thalassemia ( 100 ). One study examined whether measures of oxidative stress can be ameliorated after treatment with curcumin in patients with β-thalassemia ( 67 ). Twenty-one patients were given curcuminoids (500 mg/d) for 12 months. Blood was collected every 2 months during treatment and 3 months after withdrawal and was analyzed for MDA, superoxide dismutase, glutathione peroxidase (GSH-Px), and reduced GSH in RBCs, as well as non-transferrin-bound iron in serum. An increase in oxidative stress was reported, as indicated by higher levels of MDA, superoxide dismutase, and GSH-Px in RBCs, higher non-transferrin-bound iron in serum, and lower levels of RBC GSH in patients. Curcuminoid administration was associated with improvement in these measures. Furthermore, 3 months after withdrawal of curcuminoid treatment, all measures returned close to baseline levels. The authors of this study concluded that curcuminoids may be used to ameliorate oxidative damage in patients with β-thalassemia. However, further studies are required to demonstrate whether improvement in these parameters by curcuminoids is associated with improvement in symptoms of β-thalassemia.
Biliary Dyskinesia
Biliary dyskinesia is a motility disorder that affects the gallbladder and sphincter of Oddi. The disease is often associated with right upper abdominal pain. A multicenter pilot study analyzed the effects of dried Curcuma extracts on abdominal pain in the right upper quadrant due to biliary dyskinesia ( 68 ). The extract was given to 39 patients and placebo to 37 patients for 3 weeks. Pain reduction was more rapid during the first treatment week in patients who received the extract than in the control group. Secondary variables such as food intolerance, nausea, vomiting, and meteorism were also improved in the extract-treated patients during the whole treatment period, and the extract was not associated with any adverse effects. Thus, this study provides evidence for the beneficial effects of Curcuma extract on pain due to biliary dyskinesia ( 68 ). However, how the extract mediates pain-relieving activity remains to be investigated.
Gallbladder Contraction
The gallbladder is a very small but important organ that serves as a reservoir for bile, which it helps to release into the small intestine to digest fats. The need for bile in the small intestine is signaled by a hormone called cholecystokinin, which causes the gallbladder to contract and deliver bile into the intestine. Alterations in gallbladder contraction may contribute to pathological conditions such as cholesterol gallstone formation and cholecystitis. Gallbladder contraction can be stimulated by using synthetic hormones such as cholecystokinin, caerulein, and motilin and cholinomimetic drugs such as bethanechol, neostigmine, and erythromycin ( 101 ). A randomized, double-blind, crossover study compared the effect of 20 mg of curcumin or placebo on the gallbladder volume of 12 healthy volunteers ( 69 ). Ultrasonographic examination was carried out serially to measure the gallbladder volume. The gallbladder volume was reduced within the period after curcumin administration. The percentages of gallbladder volume reduction at 0.5, 1, 1.5, and 2 h after curcumin administration were 11.8%, 16.8%, 22%, and 29.3%, respectively. These results suggest the ability of curcumin in stimulating gallbladder contraction and reducing the risk of gallstones formation ( 69 ). Further dose–response studies are required to determine the optimal dose of curcumin that can induce further increase in contraction.
Recurrent Respiratory Tract Infections
Recurrent respiratory tract infections (RRTIs) are common diseases in childhood and constitute a serious problem worldwide. The anti-inflammatory or anti-bacterial drugs used for these infections are often associated with adverse effects and contribute to the selection of drug-resistant microorganisms. One study examined the clinical and immunologic effects of lactoferrin and curcumin (LC) oral supplementation in healthy children with RRTIs ( 70 ). Ten children with RRTIs received LC orally at 1 g (900 mg lactoferrin plus 100 mg curcumin) every 8 h for 4 weeks. Administration of LC was associated with reduction in RRTIs and beneficial immune-modulatory effects in children. Randomized clinical trials will further validate the possible effects of LC in reducing RRTIs.
ATT-Induced Hepatotoxicity
Although isoniazid, rifampicin, pyrazinamide, and ethambutol are used as anti-tuberculosis treatment (ATT), the associated hepatotoxicity represents a major disadvantage. The exact mechanism of ATT-induced hepatotoxicity is unknown, but oxidative stress, choline deficiency, reduced glutathione level, and activation of CYP2E1 may play crucial roles. Occurrences of such hepatotoxicity are normally managed by stopping the drug use and reintroducing the same drug after normalization of liver enzymes. Adhvaryu et al . ( 71 ) conducted a randomized controlled clinical trial to evaluate the efficacy of C. longa in controlling hepatotoxic episodes in patients with a diagnosis of tuberculosis who were undergoing ATT. A total of 528 patients participated in the study. The patients were randomly assigned to a control group (200patients) and a trial group (328patients), from which 192 and 316 patients, respectively, completed the study. The ATT consisted of isoniazid, rifampicin, pyrazinamide, and ethambutol for the first 2 months followed by continuation phase therapy that excluded pyrazinamide for 4 months. In the treatment group, patients were given curcumin-enriched (25%) C. longa and Tinospora extract (1 g/d each) along with ATT. Only 2 of the 316 patients from the trial group and 27 of 192 patients from the control group developed hepatotoxicity. There were increases in liver aspartate transaminase (AST), alanine transaminase (ALT), and bilirubin concentrations in the control group but not in the treatment group. Furthermore, significant weight gain and a decreased erythrocyte sedimentation rate were observed in the treatment group compared with controls (Fig. 6b ). The authors of this study concluded that C. longa can be used as an adjuvant to prevent ATT-associated hepatotoxicity. However, more clinical trials are required to determine the effectiveness of curcumin in latent tuberculosis cases and multidrug-resistant cases.
Chronic Arsenic Exposure
Groundwater arsenic contamination is a global threat to human health and is associated with carcinogenic effects. The biggest cases of groundwater arsenic contamination can be found in Bangladesh and in West Bengal in India. The carcinogenic effects of arsenic are likely mediated through oxidative DNA damage. Therefore, agents with antioxidant capacity may have potential against arsenic-induced genotoxic effects. A field trial from West Bengal evaluated the role of curcumin against the genotoxic effects of arsenic ( 72 ). A total of 286 volunteers exposed to groundwater arsenic were recruited into the study. The participants were randomly assigned to a placebo group (143 persons) and a curcumin-treated group (143 persons). Curcumin was given at a dose of 500 mg twice daily for 3 months in combination with piperine. DNA damage in lymphocytes was assessed by the comet assay and fluorescence-activated DNA unwinding assay. Curcumin was analyzed in blood by high-performance liquid chromatography. Arsenic-induced oxidative stress and curcumin’s antagonistic role were evaluated by measuring reactive oxygen species (ROS) generation, lipid peroxidation, and protein carbonyl contents. The blood samples from this arsenic-exposed population showed severe DNA damage with increased levels of ROS and lipid peroxidation. Three months of curcumin intervention reduced the DNA damage and retarded ROS generation and lipid peroxidation. Curcumin treatment was also associated with significant enhancement in the levels of such anti-oxidants as catalase, superoxide dismutase, glutathione peroxidase, and glutathione. The authors of this study concluded that curcumin may have some protective role against arsenic-induced DNA damage ( 72 ).
Alcohol Intoxication
Theracurmin, a highly absorptive curcumin dispersed with colloidal nanoparticles, was recently shown to exhibit an inhibitory action against alcohol intoxication in humans ( 73 ).
Chronic Bacterial Prostatitis
Chronic bacterial prostatitis (CBP) is a persistent infection of the prostate gland characterized by poor quality of life. Both gram-negative ( 102 ) and gram-positive bacteria are involved in the pathogenesis of CBP ( 103 ). Current treatments for CBP include use of antibiotics that penetrate the prostate and kill the causative organisms. However, poor penetration of antibiotics to the prostate tissue, drug resistance of uropathogens, and the adverse effects associated with antibiotic treatment necessitate alternative approaches for CBP treatment. A randomized, long-term follow-up study evaluated the efficacy of combinations of Serenoarepens (160 mg) plus Urticadioica (120 mg) (ProstaMEV®), and curcumin (200 mg) plus quercetin (100 mg) (FlogMEV®) in improving the efficacy of prulifloxacin in patients with CBP ( 74 ). A total of 143 CBP patients divided into two groups (A and B) were enrolled in this study and received prulifloxacin (600 mg) daily for 14 days. A total of 106 patients in group A received prulifloxacin in combination with ProstaMEV® and FlogMEV® whereas 37 patients in group B received only antibiotic therapy. One month after treatment, 89.6% of patients in group A reported no symptoms associated with CBP, whereas only 27% of patients who received antibiotic therapy alone were recurrence-free. Significant differences were found between groups in terms of symptoms and improvements in quality of life. Six months after treatment, no patients in group A had recurrence of disease, whereas two patients in group B did. It was concluded that ProstaMEV® and FlogMEV® can improve the clinical efficacy of prulifloxacin in patients with CBP ( 74 ). However, the relative contribution of curcumin in improving CBP symptoms was not evaluated.
ONGOING CLINICAL TRIALS
Although numerous clinical trials have been completed, some are still evaluating the efficacy of curcumin against human ailments. A search on www.clinicaltrials.gov (accessed in February 2012) indicated that about 35 clinical trials with curcumin are ongoing. The most common human diseases for which curcumin is being evaluated are cancer, IrBS, inflammatory conditions, arthritis, neurological conditions, and diabetes (Table III ). Although curcumin is being evaluated all over the world, most of these clinical trials are from the United States. A team from the University of Leicester, UK, and Cancer Research UK has planned to initiate a clinical trial that will examine whether curcumin can improve response to chemotherapy (FOLFOX; combinations of 5-FU, leucovorin, and oxaliplatin) in patients with advanced bowel cancer ( 104 ). This 2-year trial will recruit 42 patients with bowel cancer that has metastasized to the liver; 75% will receive curcumin for 7 days, followed by FOLFOX, and the remainder will receive FOLFOX only. Some clinical trials, such as one from India for Alzheimer’s disease and one from James Graham Brown Cancer Center in the United States are still recruiting patients. The estimated primary completion times for most of these ongoing clinical trials range from 6 months to 10 years. These trials for various diseases are in different phases and are using curcumin mostly in the form of nanoparticles, capsules, tablets, powder, and solutions. Doses ranging from 0.18 to 8 g/day are being used in these trials. For some diseases, curcumin is being administered in combination with other agents and therapies such as chemotherapeutics, radiation, and other nutraceuticals. These ongoing clinical trials are expected to provide a deeper understanding of curcumin’s efficacy and mechanism of action against human diseases.
Ongoing Clinical Trials with Curcumin
ESRF end-stage renal failure, LH optic neuropathy Leber’s hereditary optic neuropathy, NSCLC non–small cell lung cancer, JHU Johns Hopkins University, LHRI Lawson Health Research Institute, LSUHSC Louisiana State University Health Science Center, MU Mahidol University, SU Srinakharinwirot University, TASMC Tel Aviv Sourasky Medical Center, UH University Hospitals, UNC University of North Carolina, UTMDACC The University of Texas MD Anderson Cancer Center
www.clinicaltrials.gov
a Given in combination with other drugs
ADVERSE EVENTS ASSOCIATED WITH CURCUMIN
Although curcumin has been shown to exhibit beneficial activities in a plethora of human diseases with minimal toxicities, some investigators have reported undesired adverse effects associated with this polyphenol. Lao et al. ( 105 ) conducted a dose-escalation study to determine the maximum tolerable dose and safety of a single oral dose of curcumin in 34 healthy volunteers. The volunteers were given escalating doses of curcumin ranging from 500 to 12,000 mg, and safety was assessed for 72 h after administration. Twenty-four participants completed the trial, seven of whom experienced minimal toxicity that did not appear to be dose-related. More specifically, these seven participants experienced diarrhea, headache, rash, and yellow stool.
In another study, curcumin at doses ranging from 0.45 to 3.6 g/day for 1 to 4 months was associated with nausea and diarrhea and caused an increase in serum alkaline phosphatase and lactate dehydrogenase contents in human participants ( 14 ). In patients with high-risk or premalignant lesions, doses of curcumin above 8 g/day were unacceptable to patients because of the bulky volume of the tablets ( 28 ). In one study of patients with advanced pancreatic cancer, 5 of the 17 patients receiving curcumin (8 g/day) in combination with gemcitabine reported intractable abdominal pain after a few days to 2 weeks of curcumin intake ( 20 ). Thus, more studies are required to evaluate the long-term toxicity associated with curcumin before it can be approved for human use.
CONCLUSIONS
Subsequent to the first seminal paper published in 1949 in Nature , numerous preclinical studies have provided a solid basis for examining curcumin’s efficacy against human diseases. As discussed in this review, curcumin has shown therapeutic potential against a number of human diseases. Common to all of these studies have been the safety, tolerability, and non-toxicity of this polyphenol, even at doses up to 8 g per day. The underlying mechanism for curcumin’s clinical efficacy seems to be modulation of numerous signaling molecules. However, because of the complex nature of the diseases, the underlying mechanism in many cases remains unclear.
From the findings of the completed clinical trials, it may seem that curcumin’s clinical efficacy is too good to be true. However, this polyphenol has not yet been approved for human use. Poor bioavailability and limited adverse effects reported by some investigators are a major limitation to the therapeutic utility of curcumin. We hope that the results from ongoing clinical trials will provide a deeper understanding of curcumin’s therapeutic potential and will help to place this fascinating molecule at the fore front of novel therapeutics.
Acknowledgments
We thank Michael Worley, Tamara K. Locke and the Department of Scientific Publications for carefully editing the manuscript and providing valuable comments. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research.
Invited review for The AAPS Journal
Editor-in-Chief: Ho-Leung Fung, Ph.D.
- 1. Frantz S. Drug discovery: playing dirty. Nature. 2005;437(7061):942–943. doi: 10.1038/437942a. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Mencher SK, Wang LG. Promiscuous drugs compared to selective drugs (promiscuity can be a virtue) BMC Clin Pharmacol. 2005;5(1):3. doi: 10.1186/1472-6904-5-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Vogel A, Pelletier J. Examen chimique de la racine de Curcuma. J Pharm. 1815;1:289–300. [ Google Scholar ]
- 4. Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39(3):283–299. doi: 10.1111/j.1440-1681.2011.05648.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Schraufstatter E, Bernt H. Antibacterial action of curcumin and related compounds. Nature. 1949;164(4167):456. doi: 10.1038/164456a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30(2):85–94. doi: 10.1016/j.tips.2008.11.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41(1):40–59. doi: 10.1016/j.biocel.2008.06.010. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, et al. Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep. 2011;28(12):1937–1955. doi: 10.1039/c1np00051a. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Loeber CC. De curcuma officinarum. diss Inaug Halae. 1748.
- 10. Oppenheimer A. Turmeric (curcumin) in biliary diseases. Lancet. 1937;229:619–621. doi: 10.1016/S0140-6736(00)98193-5. [ DOI ] [ Google Scholar ]
- 11. Kanai M, Yoshimura K, Asada M, Imaizumi A, Suzuki C, Matsumoto S, et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol. 2011;68(1):157–164. doi: 10.1007/s00280-010-1470-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14(14):4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7(7):1894–1900. [ PubMed ] [ Google Scholar ]
- 14. Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10(20):6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Garcea G, Berry DP, Jones DJ, Singh R, Dennison AR, Farmer PB, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005;14(1):120–125. [ PubMed ] [ Google Scholar ]
- 16. Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4(8):1035–1038. doi: 10.1016/j.cgh.2006.03.020. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 2011;4(3):354–364. doi: 10.1158/1940-6207.CAPR-10-0098. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. He ZY, Shi CB, Wen H, Li FL, Wang BL, Wang J. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Investig. 2011;29(3):208–213. doi: 10.3109/07357907.2010.550592. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Durgaprasad S, Pai CG, Vasanthkumar, Alvres JF, Namitha S. A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J Med Res. 2005;122(4):315–318. [ PubMed ] [ Google Scholar ]
- 20. Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar-Sela G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer. 2010;62(8):1137–1141. doi: 10.1080/01635581.2010.513802. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Bayet-Robert M, Kwiatkowski F, Leheurteur M, Gachon F, Planchat E, Abrial C, et al. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther. 2010;9(1):8–14. doi: 10.4161/cbt.9.1.10392. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Ide H, Tokiwa S, Sakamaki K, Nishio K, Isotani S, Muto S, et al. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate. 2010;70(10):1127–1133. doi: 10.1002/pros.21147. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Golombick T, Diamond TH, Badmaev V, Manoharan A, Ramakrishna R. The potential role of curcumin in patients with monoclonal gammopathy of undefined significance–its effect on paraproteinemia and the urinary N-telopeptide of type I collagen bone turnover marker. Clin Cancer Res. 2009;15(18):5917–5922. doi: 10.1158/1078-0432.CCR-08-2217. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Vadhan-Raj S, Weber D, Wang M, Giralt S, Alexanian R, Thomas S, et al. Curcumin downregulates NF-КB and related genes in patients with multiple myeloma: results of a phase 1/2 study. Blood. 2007;110(11):357a. [ Google Scholar ]
- 25. Polasa K, Raghuram TC, Krishna TP, Krishnaswamy K. Effect of turmeric on urinary mutagens in smokers. Mutagenesis. 1992;7(2):107–109. doi: 10.1093/mutage/7.2.107. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori. 1987;73(1):29–31. doi: 10.1177/030089168707300105. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Hastak K, Lubri N, Jakhi SD, More C, John A, Ghaisas SD, et al. Effect of turmeric oil and turmeric oleoresin on cytogenetic damage in patients suffering from oral submucous fibrosis. Cancer Lett. 1997;116(2):265–269. doi: 10.1016/S0304-3835(97)00205-X. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–2900. [ PubMed ] [ Google Scholar ]
- 29. Chainani-Wu N, Silverman S, Jr, Reingold A, Bostrom A, Mc Culloch C, Lozada-Nur F, et al. A randomized, placebo-controlled, double-blind clinical trial of curcuminoids in oral lichen planus. Phytomedicine. 2007;14(7–8):437–446. doi: 10.1016/j.phymed.2007.05.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Rai B, Kaur J, Jacobs R, Singh J. Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J Oral Sci. 2010;52(2):251–256. doi: 10.2334/josnusd.52.251. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Kim SG, Veena MS, Basak SK, Han E, Tajima T, Gjertson DW, et al. Curcumin treatment suppresses IKKbeta kinase activity of salivary cells of patients with head and neck cancer: a pilot study. Clin Cancer Res. 2011;17(18):5953–5961. doi: 10.1158/1078-0432.CCR-11-1272. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50(11):2191–2193. doi: 10.1007/s10620-005-3032-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4(12):1502–1506. doi: 10.1016/j.cgh.2006.08.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Lahiff C, Moss AC. Curcumin for clinical and endoscopic remission in ulcerative colitis. Inflamm Bowel Dis. 2011;17(7):E66. doi: 10.1002/ibd.21710. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Epstein J, Docena G, MacDonald TT, Sanderson IR. Curcumin suppresses p38 mitogen-activated protein kinase activation, reduces IL-1beta and matrix metalloproteinase-3 and enhances IL-10 in the mucosa of children and adults with inflammatory bowel disease. Br J Nutr. 2010;103(6):824–832. doi: 10.1017/S0007114509992510. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Bundy R, Walker AF, Middleton RW, Booth J. Turmeric extract may improve irritable bowel syndrome symptomology in otherwise healthy adults: a pilot study. J Altern Complement Med. 2004;10(6):1015–1018. doi: 10.1089/acm.2004.10.1015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Shimouchi A, Nose K, Takaoka M, Hayashi H, Kondo T. Effect of dietary turmeric on breath hydrogen. Dig Dis Sci. 2009;54(8):1725–1729. doi: 10.1007/s10620-008-0550-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Deodhar SD, Sethi R, Srimal RC. Preliminary study on antirheumatic activity of curcumin (diferuloyl methane) Indian J Med Res. 1980;71:632–634. [ PubMed ] [ Google Scholar ]
- 39. Chandran B, Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res. 2012;26(11):1719–25. doi:10.1002/ptr.4639. [ DOI ] [ PubMed ]
- 40. Belcaro G, Cesarone MR, Dugall M, Pellegrini L, Ledda A, Grossi MG, et al. Product-evaluation registry of Meriva(R), a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med. 2010;52(2 Suppl 1):55–62. [ PubMed ] [ Google Scholar ]
- 41. Belcaro G, Cesarone MR, Dugall M, Pellegrini L, Ledda A, Grossi MG, et al. Efficacy and safety of Meriva(R), a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern Med Rev. 2010;15(4):337–344. [ PubMed ] [ Google Scholar ]
- 42. Lal B, Kapoor AK, Asthana OP, Agrawal PK, Prasad R, Kumar P, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13(4):318–322. doi: 10.1002/(SICI)1099-1573(199906)13:4<318::AID-PTR445>3.0.CO;2-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Allegri P, Mastromarino A, Neri P. Management of chronic anterior uveitis relapses: efficacy of oral phospholipidic curcumin treatment. Long-term follow-up. Clin Ophthalmol. 2010;4:1201–1206. doi: 10.2147/OPTH.S13271. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol. 1986;24(12):651–654. [ PubMed ] [ Google Scholar ]
- 45. Kositchaiwat C, Kositchaiwat S, Havanondha J. Curcuma longa Linn. in the treatment of gastric ulcer comparison to liquid antacid: a controlled clinical trial. J Med Assoc Thail. 1993;76(11):601–605. [ PubMed ] [ Google Scholar ]
- 46. Prucksunand C, Indrasukhsri B, Leethochawalit M, Hungspreugs K. Phase II clinical trial on effect of the long turmeric (Curcuma longa Linn) on healing of peptic ulcer. Southeast Asian J Trop Med Public Health. 2001;32(1):208–215. [ PubMed ] [ Google Scholar ]
- 47. Di Mario F, Cavallaro LG, Nouvenne A, Stefani N, Cavestro GM, Iori V, et al. A curcumin-based 1-week triple therapy for eradication of Helicobacter pylori infection: something to learn from failure? Helicobacter. 2007;12(3):238–243. doi: 10.1111/j.1523-5378.2007.00497.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Koosirirat C, Linpisarn S, Changsom D, Chawansuntati K, Wipasa J. Investigation of the anti-inflammatory effect of Curcuma longa in Helicobacter pylori-infected patients. Int Immunopharmacol. 2010;10(7):815–818. doi: 10.1016/j.intimp.2010.04.021. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Lal B, Kapoor AK, Agrawal PK, Asthana OP, Srimal RC. Role of curcumin in idiopathic inflammatory orbital pseudotumours. Phytother Res. 2000;14(6):443–447. doi: 10.1002/1099-1573(200009)14:6<443::AID-PTR619>3.0.CO;2-V. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Asawanonda P, Klahan SO. Tetrahydrocurcuminoid cream plus targeted narrowband UVB phototherapy for vitiligo: a preliminary randomized controlled study. Photomed Laser Surg. 2010;28(5):679–684. doi: 10.1089/pho.2009.2637. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Heng MC, Song MK, Harker J, Heng MK. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol. 2000;143(5):937–949. doi: 10.1046/j.1365-2133.2000.03767.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Kurd SK, Smith N, VanVoorhees A, Troxel AB, Badmaev V, Seykora JT, et al. Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: a prospective clinical trial. J Am Acad Dermatol. 2008;58(4):625–631. doi: 10.1016/j.jaad.2007.12.035. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Burns J, Joseph PD, Rose KJ, Ryan MM, Ouvrier RA. Effect of oral curcumin on Dejerine-Sottas disease. Pediatr Neurol. 2009;41(4):305–308. doi: 10.1016/j.pediatrneurol.2009.04.030. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL. A potential role of the curry spice curcumin in Alzheimer’s disease. Curr Alzheimer Res. 2005;2(2):131–136. doi: 10.2174/1567205053585882. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008;28(1):110–113. doi: 10.1097/jcp.0b013e318160862c. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Alwi I, Santoso T, Suyono S, Sutrisna B, Suyatna FD, Kresno SB, et al. The effect of curcumin on lipid level in patients with acute coronary syndrome. Acta Med Indones. 2008;40(4):201–210. [ PubMed ] [ Google Scholar ]
- 57. Soni KB, Kuttan R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J Physiol Pharmacol. 1992;36(4):273–275. [ PubMed ] [ Google Scholar ]
- 58. Srinivasan M. Effect of curcumin on blood sugar as seen in a diabetic subject. Indian J Med Sci. 1972;26(4):269–270. [ PubMed ] [ Google Scholar ]
- 59. Usharani P, Mateen AA, Naidu MU, Raju YS, Chandra N. Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: a randomized, parallel-group, placebo-controlled, 8-week study. Drugs R D. 2008;9(4):243–250. doi: 10.2165/00126839-200809040-00004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Wickenberg J, Ingemansson SL, Hlebowicz J. Effects of Curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutr J. 2010;9:43. doi: 10.1186/1475-2891-9-43. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. 2012;35(11):2121–7. doi:10.2337/dc12-0116. [ DOI ] [ PMC free article ] [ PubMed ]
- 62. Khajehdehi P, Pakfetrat M, Javidnia K, Azad F, Malekmakan L, Nasab MH, et al. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-beta and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study. Scand J Urol Nephrol. 2011;45(5):365–370. doi: 10.3109/00365599.2011.585622. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Appendino G, Belcaro G, Cornelli U, Luzzi R, Togni S, Dugall M, et al. Potential role of curcumin phytosome (Meriva) in controlling the evolution of diabetic microangiopathy. A pilot study. Panminerva Med. 2011;53(3 Suppl 1):43–49. [ PubMed ] [ Google Scholar ]
- 64. Khajehdehi P, Zanjaninejad B, Aflaki E, Nazarinia M, Azad F, Malekmakan L, et al. Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: a randomized and placebo-controlled study. J Ren Nutr. 2012;22(1):50–57. doi: 10.1053/j.jrn.2011.03.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Shoskes D, Lapierre C, Cruz-Correa M, Muruve N, Rosario R, Fromkin B, et al. Beneficial effects of the bioflavonoids curcumin and quercetin on early function in cadaveric renal transplantation: a randomized placebo controlled trial. Transplantation. 2005;80(11):1556–1559. doi: 10.1097/01.tp.0000183290.64309.21. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. James JS. Curcumin: clinical trial finds no antiviral effect. AIDS Treat News. 1996;(no 242):1–2. [ PubMed ]
- 67. Kalpravidh RW, Siritanaratkul N, Insain P, Charoensakdi R, Panichkul N, Hatairaktham S, et al. Improvement in oxidative stress and antioxidant parameters in beta-thalassemia/Hb E patients treated with curcuminoids. Clin Biochem. 2010;43(4–5):424–429. doi: 10.1016/j.clinbiochem.2009.10.057. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Niederau C, Gopfert E. The effect of chelidonium- and turmeric root extract on upper abdominal pain due to functional disorders of the biliary system. Results from a placebo-controlled double-blind study. Med Klin (Munich) 1999;94(8):425–430. doi: 10.1007/BF03044726. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Rasyid A, Lelo A. The effect of curcumin and placebo on human gall-bladder function: an ultrasound study. Aliment Pharmacol Ther. 1999;13(2):245–249. doi: 10.1046/j.1365-2036.1999.00464.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Zuccotti GV, Trabattoni D, Morelli M, Borgonovo S, Schneider L, Clerici M. Immune modulation by lactoferrin and curcumin in children with recurrent respiratory infections. J Biol Regul Homeost Agents. 2009;23(2):119–123. [ PubMed ] [ Google Scholar ]
- 71. Adhvaryu MR, Reddy N, Vakharia BC. Prevention of hepatotoxicity due to anti tuberculosis treatment: a novel integrative approach. World J Gastroenterol. 2008;14(30):4753–4762. doi: 10.3748/wjg.14.4753. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Biswas J, Sinha D, Mukherjee S, Roy S, Siddiqi M, Roy M. Curcumin protects DNA damage in a chronically arsenic-exposed population of West Bengal. Hum Exp Toxicol. 2010;29(6):513–524. doi: 10.1177/0960327109359020. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, et al. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull. 2011;34(5):660–665. doi: 10.1248/bpb.34.660. [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Cai T, Mazzoli S, Bechi A, Addonisio P, Mondaini N, Pagliai RC, et al. Serenoa repens associated with urtica dioica (ProstaMEV) and curcumin and quercitin (FlogMEV) extracts are able to improve the efficacy of prulifloxacin in bacterial prostatitis patients: results from a prospective randomised study. Int J Antimicrob Agents. 2009;33(6):549–553. doi: 10.1016/j.ijantimicag.2008.11.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–356. doi: 10.1055/s-2006-957450. [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Gota VS, Maru GB, Soni TG, Gandhi TR, Kochar N, Agarwal MG. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J Agric Food Chem. 2010;58(4):2095–2099. doi: 10.1021/jf9024807. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Cuomo J, Appendino G, Dern AS, Schneider E, McKinnon TP, Brown MJ, et al. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J Nat Prod. 2011;74(4):664–669. doi: 10.1021/np1007262. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S. A pilot cross-over study to evaluate human oral bioavailability of BCM-95CG (Biocurcumax), a novel bioenhanced preparation of curcumin. Indian J Pharm Sci. 2008;70(4):445–449. doi: 10.4103/0250-474X.44591. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. Disilvestro RA, Joseph E, Zhao S, Joshua B. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr J. 2012;11(1):79. doi: 10.1186/1475-2891-11-79. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 81. Goel A, Jhurani S, Aggarwal BB. Multi-targeted therapy by curcumin: how spicy is it? Mol Nutr Food Res. 2008;52(9):1010–1030. doi: 10.1002/mnfr.200700354. [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29(3):405–434. doi: 10.1007/s10555-010-9235-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 83. Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford) 2008;10(1):58–62. doi: 10.1080/13651820701883148. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance. Clin Lymphoma Myeloma. 2005;6(2):102–114. doi: 10.3816/CLM.2005.n.036. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Scully C, Beyli M, Ferreiro MC, Ficarra G, Gill Y, Griffiths M, et al. Update on oral lichen planus: etiopathogenesis and management. Crit Rev Oral Biol Med. 1998;9(1):86–122. doi: 10.1177/10454411980090010501. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15(2):425–430. doi: 10.1158/1078-0432.CCR-08-0149. [ DOI ] [ PubMed ] [ Google Scholar ]
- 87. Megraud F, Brassens-Rabbe MP, Denis F, Belbouri A, Hoa DQ. Seroepidemiology of Campylobacter pylori infection in various populations. J Clin Microbiol. 1989;27(8):1870–1873. doi: 10.1128/jcm.27.8.1870-1873.1989. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Birch-Hirschfeld A. Zur diagnostic and pathologic der orbital tumoren. Ber Dtsch Opthalmol Ges. 1905;32:127–135. [ Google Scholar ]
- 89. Orcutt JC, Garner A, Henk JM, Wright JE. Treatment of idiopathic inflammatory orbital pseudotumours by radiotherapy. Br J Ophthalmol. 1983;67(9):570–574. doi: 10.1136/bjo.67.9.570. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 90. Schallreuter KU, Wood JM, Pittelkow MR, Buttner G, Swanson N, Korner C, et al. Increased monoamine oxidase A activity in the epidermis of patients with vitiligo. Arch Dermatol Res. 1996;288(1):14–18. doi: 10.1007/BF02505037. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Arca E, Tastan HB, Erbil AH, Sezer E, Koc E, Kurumlu Z. Narrow-band ultraviolet B as monotherapy and in combination with topical calcipotriol in the treatment of vitiligo. J Dermatol. 2006;33(5):338–343. doi: 10.1111/j.1346-8138.2006.00079.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741. doi: 10.1001/jama.296.14.1735. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Reddy S, Aggarwal BB. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Lett. 1994;341(1):19–22. doi: 10.1016/0014-5793(94)80232-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Mattson MP, Rydel RE. Alzheimer’s disease. Amyloid ox-tox transducers. Nature. 1996;382(6593):674–675. doi: 10.1038/382674a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Goyal A, Petersen JL, Mahaffey KW. The evaluation and management of dyslipidemia and impaired glucose metabolism during acute coronary syndromes. Curr Cardiol Rep. 2004;6(4):300–307. doi: 10.1007/s11886-004-0080-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Sathyapalan T, Atkin SL. Is there a role for immune and anti-in-flammatory therapy in type 2 diabetes? Minerva Endocrinol. 2011;36(2):147–156. [ PubMed ] [ Google Scholar ]
- 98. Camera A, Hopps E, Caimi G. Diabetic microangiopathy: physiopathological, clinical and therapeutic aspects. Minerva Endocrinol. 2007;32(3):209–229. [ PubMed ] [ Google Scholar ]
- 99. Shoskes DA, Shahed AR, Kim S. Delayed graft function. Influence on outcome and strategies for prevention. Urol Clin N Am. 2001;28(4):721–732. doi: 10.1016/S0094-0143(01)80028-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Fibach E, Rachmilewitz E. The role of oxidative stress in hemolytic anemia. Curr Mol Med. 2008;8(7):609–619. doi: 10.2174/156652408786241384. [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Marzio L. Factors affecting gallbladder motility: drugs. Dig Liver Dis. 2003;35(Suppl 3):S17–S19. doi: 10.1016/S1590-8658(03)00088-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 102. Wagenlehner FM, Diemer T, Naber KG, Weidner W. Chronic bacterial prostatitis (NIH type II): diagnosis, therapy and influence on the fertility status. Andrologia. 2008;40(2):100–104. doi: 10.1111/j.1439-0272.2007.00827.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. Nickel JC, Xiang J. Clinical significance of nontraditional bacterial uropathogens in the management of chronic prostatitis. J Urol. 2008;179(4):1391–1395. doi: 10.1016/j.juro.2007.11.081. [ DOI ] [ PubMed ] [ Google Scholar ]
- 104. Rodwell C. Curcumin curries favour? Nat Rev Cancer. 2012;12(6):376. doi: 10.1038/nrc3288. [ DOI ] [ Google Scholar ]
- 105. Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (853.9 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO
COMMENTS
In addition, Mayo Clinic has a research subject advocate who is independent of all clinical studies and is a resource for research participants. Contact the research subject advocate by email or at 507-266-9372 with questions, concerns and ideas for improving research practices. Find out more about volunteering for clinical studies and how to ...
Clinical Trials Open to Healthy Volunteers. A "healthy volunteer" is someone who doesn't have any major health problems, and takes part in research to test new medicines, devices, or treatments. UCSF offers clinical trials on a wide range of medical conditions. Healthy volunteers can help UCSF researchers make better comparisons to better ...
When you select individual studies, carefully review the study overview and eligibility requirements. If you meet the eligibility requirements, call 1-800-411-1222 (TTY 1-866-411-1010). We can provide participation details on up to three studies a day. Ask about joining our Clinical Research Volunteer Program registry.
ResearchMatch helps you find a clinical trial or research study near you, or across the country, by matching you with researchers from leading medical research institutions. Whether you are a healthy volunteer or have a health condition, ResearchMatch connects you to research opportunities so you can make a difference and advance scientific discoveries by participating in research studies ...
ResearchMatch helps you find a clinical trial or research study near you, or across the country, by matching you with researchers from leading medical research institutions. Whether you are a healthy volunteer or have a health condition, ResearchMatch connects you to research opportunities so you can make a difference and advance scientific discoveries by participating in research studies ...
Biotrial offers a meaningful way to spend your time while earning money. Becoming a paid clinical trial volunteer is easy! Just register to be a participant for one of our online research studies and you can start getting paid during your first screening appointment. By participating in our healthy studies, you offer hope for many people as you ...
Interested in participating in a clinical study or trial? Research volunteers help doctors and scientists to test new drugs, therapies, medical devices and clinical and surgical methods. With your help, investigators can help to treat and cure medical conditions and diseases. Whether you are a healthy patient, or someone looking to explore alternative treatments for an illness
To join the healthy volunteer registry, you will be asked to provide basic information including: your contact details ; your characteristics and health; permission for us to share your information with the clinical research study teams. If you are a potential match for a study's requirements, the clinical research study team may contact you.
A healthy volunteer receives an experimental universal influenza vaccine known as H1ssF_3928 as part of a Phase 1 clinical trial at the NIH Clinical Center in Bethesda, Maryland. Scientists at NIAID's Vaccine Research Center (VRC) developed the vaccine and are leading the clinical trial.
If you are interested in any of the research below, please contact us at [email protected] or 407 COM TRIALS (266 8742). Join The Contact Registry For Clinical Research. Sign up for the UCF College of Medicine's Contact Registry for Clinical Trials to learn about clinical research and clinical trials in areas of interest to you.
Clinical trials are a key research tool for advancing medical knowledge and patient care. They're run to test various scientific developments - including medical interventions, surgical and radiological procedures, new devices, behavioral treatments, and preventive care. However, a recent study by the Health Information National Trends ...
Experienced Recruitment For Early Clinical Development. Celerion has an unbeatable record for study subject recruitment, both in healthy and patient populations. The goal is to find subjects and quickly get them screened and registered for your study. Our expert recruitment and call center teams are renowned in the industry for starting studies ...
The goal of clinical trials is to determine if a new test or treatment works and is safe. Clinical trials can also look at other aspects of care, such as improving the quality of life for people with chronic illnesses. People participate in clinical trials for a variety of reasons. Healthy volunteers say they participate to help others and to ...
Steps for a Clinical Trial Volunteer. The first step to participating in a clinical trial is finding one that matches your interests, health status and geographic location. On ClinicalTrials.gov, there are currently 367,057 registered research studies in all 50 U.S. states and in 219 countries, including more than 4,500 for COVID-19.
A clinical trial is a research study in which volunteers receive investigational treatments under the supervision of a physician and other research professionals. These treatments are developed by pharmaceutical and biotechnology companies who select qualified physicians, also known as investigators, to conduct clinical trials to determine the ...
Idaho is currently home to 368 active clinical trials, seeking participants for engagement in research studies. These trials take place at a variety of cities in the state, including Boise, Meridian, Idaho Falls and Nampa.Whether you're a healthy volunteer interested in paid medical research or someone seeking trials related to a specific condition, the state offers a diverse array of ...
Treatment Clinical Trials . People with cancer: Tests new medicines, surgical procedures, and combining current treatments: Take a medicine, have surgery, get radiation. CAR-T cell therapy to treat cancers : Prevention Clinical Trials . People who had cancer in the past, and healthy volunteers* Examines how to reduce risk of getting cancer or ...
Clinical Trials Information; Find a Clinical Trial; How to Participate in Cancer Research. Everyone can support cancer research by joining a study, donating tissue, and volunteering. ... Prevention trials need healthy people and those who no longer have cancer. Cancer Screening Trials. Healthy volunteers can help to detect cancer early in ...
ResearchMatch helps you find a clinical trial or research study near you, or across the country, by matching you with researchers from leading medical research institutions. Whether you are a healthy volunteer or have a health condition, ResearchMatch connects you to research opportunities so you can make a difference and advance scientific discoveries by participating in research studies ...
Wendie Silverman-Martin, RN, Clinical Research Nurse "I like the fact that we really work well together as a team, and I enjoy contributing my intravenous and nursing skills to these studies. Our Clinical Research Team appreciates the volunteers very much - our learning about various aspects of aging depends on them.
Challenges faced by Indian investigators in subject recruitment process (n = 73)Potential strategies to enhance subject recruitment. About 63% of participants strongly agreed that creating a positive awareness about clinical trials among people through press and media would enhance recruitment followed by having a dedicated CRC for trial (50.7%) and designing a recruitment strategy prior to ...
Clinical trials have been performed to evaluate the efficacy of αGPC for treating cognitive decline in patients with Alzheimer's disease ... by the Ethics Committee of the RIKEN Center for Biosystems Dynamics Research (Kobe2 2018-02) (19 June 2018). Forty volunteers (31 females, 9 males) aged 22-59 years were recruited between July and ...
0 %. clients globally. 0 +. Atlant Clinical is a strong multinational Contract Research Organization (CRO) offering a full range of clinical trial and relevant support services throughout the US, Europe, Russia, and Middle Asia. We are a highly professional team with extensive clinical experience (Phases I-IV) in over 15 therapeutic areas.
Each involves 50 volunteers to be monitored for 90 days. Moscow will run clinical trials of two vaccines adapted to the new COVID-19 strain (XBB1), these including bi-component Sputnik V and mono-component Sputnik Light with a modified antigen composition, as announced by Anastasia Rakova, Deputy Moscow Mayor for Social Development.
This phase I/II trial studies the best dose and side effects of peposertib and to see how well it works with avelumab and hypofractionated radiation therapy in treating patients with solid tumors and hepatobiliary malignancies that have spread to other places in the body (advanced/metastatic). Peposertib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth ...
Another high-risk trial volunteer died, and more than 100 others suffered brain bleeding or swelling. ... In clinical trials for Alzheimer's drugs, genetic tests showed that certain patients ...
Part 1: Clinical Trials Administration Intensive, FPM 40273, offered Spring and Fall quarters. This is followed by: Part 2: Follow-On Program, FPM 40272; package includes 3 online classes and the Capstone workshop (also available via distance learning for students residing out of region) for one fee.
a The interest in curcumin research in human participants has increased remarkably over the years.b Human diseases against which curcumin has exhibited activity. The safety, tolerability, and nontoxicity of curcumin at high doses are well established by human clinical trials (3,4).Our own group found that curcumin at 8 g/day in combination with gemcitabine was safe and well-tolerated in ...
For decades, clinical trials have played a key role in preventing, managing (and hopefully one day, curing) diabetes. These trials are responsible for developing tools people with diabetes rely on daily - like automated insulin delivery (AID) systems, new medications, behavioral programs, and more. Since the A1C test was developed in the mid-1970s, it became the go-to measurement in clinical ...
ResearchMatch helps you find a clinical trial or research study near you, or across the country, by matching you with researchers from leading medical research institutions. Whether you are a healthy volunteer or have a health condition, ResearchMatch connects you to research opportunities so you can make a difference and advance scientific discoveries by participating in research studies ...