
- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books

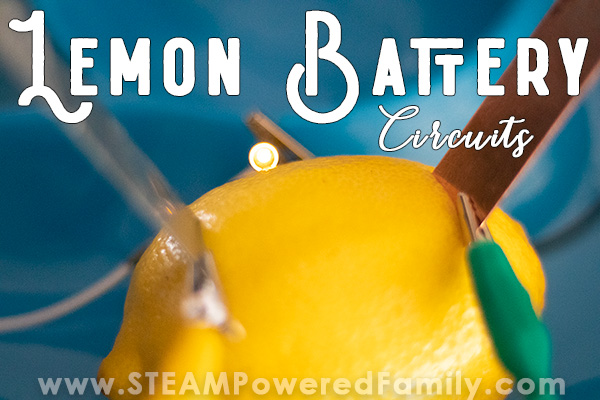
Lemon Battery Experiment

The lemon battery experiment is a classic science project that illustrates an electrical circuit, electrolytes, the electrochemical series of metals, and oxidation-reduction (redox) reactions . The battery produces enough electricity to power an LED or other small device, but not enough to cause harm, even if you touch both electrodes. Here is how to construct a lemon battery, a look at how it works, and ways of turning the project into an experiment.
Lemon Battery Materials
You need a few basic materials for a lemon battery, which are available at a grocery store and hardware store.
- Galvanized nail
- Copper penny, strip, or wire
- Wires or strips of aluminum foil
- Alligator clips or electrical tape
- An LED bulb, multimeter, digital clock, or calculator
If you don’t have a lemon, use any citrus fruit. A galvanized nail is a steel nail that is plated with zinc. The classic project uses copper and zinc because these two metals are inexpensive and readily available. However, you can use any two conductive metals, as long as they are different from each other.
Make a Lemon Battery
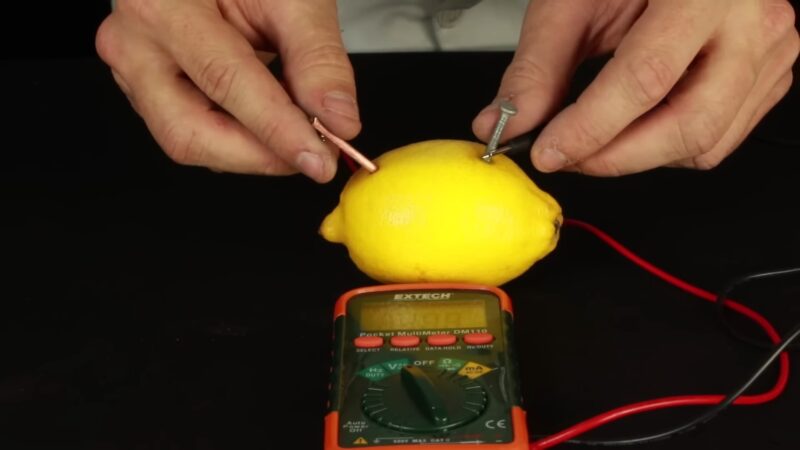
- Gently squeeze the lemon or roll it on a table to soften it. This helps the juice flow within the fruit.
- Insert the copper and zinc into the fruit. You want the maximum surface area in the juicy part of the fruit. The lemon peel helps support the metal, but if it is very thick and the metal does not reach the juice, scrape away part of the peel. Ideally, separate the metal pieces by about 2 inches (5 centimeters). Make sure the metals are not touching each other.
- Connect a wire to the galvanized nail using an alligator clip or electrical tape. Repeat the process with the copper item.
- Connect the free ends of the wire to an LED or other small electronic device. When you connect the second wire, the light turns on.
Increase the Power
The voltage of a lemon battery is around 1.3 V to 1.5 V, but it generates very little current. There are two easy ways of increasing the battery’s power.
- Use two pennies and two copper pieces in the lemon. You don’t want any of the metal pieces within the fruit to touch. As before, connect one zinc and one copper piece to the LED. But, wire the other zinc and copper to each other.
- Wire more lemons in series with each other. Insert a nail and copper piece into each nail. Connect the copper of one lemon to the zinc of the next lemon. Connect the nail at the end of the series to the LED and the copper at the end of the series to the LED. If you don’t have lots of lemons, you can cut up one lemon into pieces.

How a Lemon Battery Works
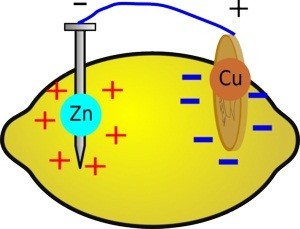
A lemon battery is similar to Volta’s first battery, except he used salt water instead of lemon juice. The zinc and copper are electrodes. The lemon juice is an electrolyte . Lemon juice contains citric acid. While both salts and acids are examples of electrolytes, acids typically do a better job in batteries.
Connecting the zinc and copper electrodes using a wire (even with an LED or multimeter between them) completes an electrical circuit. The circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc.
Zinc dissolves in lemon juice, leaving zinc ions (Zn 2+ ) in the juice, while the two electrons per atom move through the wire toward the copper. The following chemical reaction represents this oxidation reaction :
Zn → Zn 2+ + 2e −
Citric acid is a weak acid, but it partially dissociates and leaves some positively charged hydrogen ions (H + ) in the juice. The copper electrode does not dissolve. The excess electrons at the copper electrode combine with the hydrogen ions and form hydrogen gas at the copper electrode. This is a reduction reaction.
2H + + 2e − → H 2
If you perform the project using lemon juice instead of a lemon, you may observe tiny hydrogen gas bubbles forming on the copper electrode.
Try Other Fruits and Vegetables
The key for using produce in a battery is choosing a fruit of vegetable high in acid (with a low pH). Citrus fruits (lemon, orange, lime, grapefruit) contain citric acid. You don’t need a whole fruit. Orange juice and lemonade work fine. Potatoes work well because they contain phosphoric acid. Boiling potatoes before using them increases their effectiveness. Sauerkraut contains lactic acid. Vinegar works because it contains acetic acid.
Experiment Ideas
Turn the lemon battery into an experiment by applying the scientific method . Make observations about the battery, ask questions, and design experiments to test predictions or a hypothesis .
- Experiment with other materials for the electrodes besides a galvanized nail and copper item. Other common metals available in everyday life include iron, steel, aluminum, tin, and silver. Try using a nickel and a penny. What do you think will happen if you use two galvanized nails and no copper, or two pennies and no nails? What happens if you try to use plastic, wood, or glass as an electrode? Can you explain your results?
- If you have a multimeter, explore whether the distance between the electrodes affects the voltage and current of your circuit.
- How big is the effect of adding a second lemon to the circuit? Does it change the voltage? Does it change the current?
- Try making batteries using other foods from the kitchen. Predict which ones you think will work and test them. Of course, try fruits and vegetables. Also consider liquids like water, salt water, milk and juice, and condiments, like ketchup, mustard, and salsa.
The lemon battery dates back to at least 2000 years ago. Archaeologists discovered a battery in Iraq using a clay pot, lemon juice, copper, iron, and tar. Of course, people using this battery did not know about electrochemistry or even what electricity was. The use of the ancient battery is unknown.
Credit for discovery of the battery goes to Italian scientists Luigi Galvani and Alessandro Volta. In 1780, Luigi Galvani demonstrated copper, zinc, and frog legs (acting as an electrolyte) produced electricity. Galvani published his work in 1790. An electrochemical cell is called a galvanic cell in his honor.
Alessandro Volta proved electricity did not require an animal. He used brine-soaked paper as an electrolyte and invented the voltaic pile in 1799. A voltaic pile is a stack of galvanic cells, with each cell consisting of a metal disk, an electrolyte layer, and a disk of a different metal.
- Goodisman, Jerry (2001). “Observations on Lemon Cells”. Journal of Chemical Education . 78(4): 516–518. doi: 10.1021/ed078p516
- Margles, Samantha (2011). “ Does a Lemon Battery Really Work? “. Mythbusters Science Fair Book . Scholastic. ISBN 9780545237451.
- Naidu, M. S.; Kamakshiaih, S. (1995). Introduction to Electrical Engineering . Tata McGraw-Hill Education. ISBN 9780074622926.
- Schmidt, Hans-Jürgen; Marohn, Annette; Harrison, Allan G. (2007). “Factors that prevent learning in electrochemistry”. Journal of Research in Science Teaching . 44 (2): 258–283. doi: 10.1002/tea.20118
- Swartling, Daniel J.; Morgan, Charlotte (1998). “Lemon Cells Revisited—The Lemon-Powered Calculator”. Journal of Chemical Education . 75 (2): 181–182. doi: 10.1021/ed075p181
Related Posts
Science Project Ideas

How to Make a Lemon Battery
Electricity experiments are popular with the kids. However, it is not always true that electricity experiments need complex circuitry and powerful batteries. For starters, the lemon battery science project is ideal to let the young learners know the basics. Besides, they will be thrilled when low power bulbs light up as a result of their efforts. The project requires close adult supervision.

Lemon Battery Experiment
It is possible to produce electric current using the acidic properties of lemon. When you attach two electrodes to a lemon and touch your tongue to both of them at the same time, it completes an electrical circuit, making an electric current pass through the tongue, giving it a tingling sensation or a metallic taste.
- 18 gauge copper wire or a copper penny
- Steel paper clip, a small strip of zinc or a galvanized nail
- Wire stripper or clipper
- Use the wire strippers to strip off about 2.5 inches of plastic insulation from the copper wire. Then cut that piece from the main roll. This serves as the positive electrode or the cathode. If you are using a penny, make a small slit in the lemon with a knife before placing a penny snugly within it with about a third of it exposed.
- Straighten up the steel paper clip carefully. Cut the same length from it as the copper wire. Either this or the galvanized nail or zinc strip is the anode or the negative electrode.
- Use the sandpaper to make the edges of the two electrodes smooth as they would serve as the junction points of the electrical circuit. If you are using zinc as an electrode, scratch its surface lightly to expose a fresh surface.
- Roll the lemon gently on a table to loosen the soft pulp and allow the juice to flow inside. Be careful not to rupture the skin of the lemon, though.
- Insert the copper wire by 1 inch into the lemon.
- After ensuring that your tongue is moist with saliva, touch it to the copper wire. Make an observation regarding what you feel.
- Insert the paper clip, zinc strip or nail into the lemon keeping a distance of ¼th inch from the first insertion point. Ensure that the two electrodes do not touch each other at any point.
- Now, make contact with your moist tongue to both the exposed ends of the electrodes at the same time. Make an observation again regarding the sensation in your tongue.
How Many Volts Does a Lemon Battery Produce
It depends on what metal wire you are using for the anode and cathode. You can take notes of the amount of voltage produced by using different wires so you can monitor the outcome in future.
Though the voltage produced in this experiment is near about a volt that is well within safety limits, those of you who do not desire any physical contact with the equipment can test the electric current and voltage by connecting a multimeter to the electrodes. They need to connect the leads of the device with the lemon battery with the help of alligator clips.

Lemon Battery Science Fair Project Video
How does a lemon battery work.
A battery converts chemical energy into electrical energy. The electrodes here are made to come into contact with the lemon juice that is nothing but citric acid, an electrolyte. As a result, a chemical reaction ensues and electrons start to get accumulated at the anode. However, positive charges build up at the cathode. As soon as a conducting material (your tongue) establishes a connection between the electrodes, the electrons get a chance to flow from the cathode to the anode forming an electric current. When you touch your tongue to the copper wire only, before connecting the zinc strip, no reaction occurs. Hence, you feel nothing on your tongue in the absence of electric current.
Things You Can Try for Better Results
You will find that the voltage produced by a single lemon powered battery is not enough to light a small bulb or LED. However, if you connect a number of lemon batteries in series, you will find the resultant electricity sufficient to light an LED. Try that out by making around four to five lemon batteries and connecting the anode of one with the cathode of the next with a copper wire, for the entire set. Connect the LED bulb in between the copper wire and zinc strip left free at the extreme ends to see the results. A digital clock or calculator with its regular battery removed can also be made to work with this homemade battery.
You can plot a graph with the number of lemon batteries in the circuit as the independent variable (X-axis) and the voltage generated as measured by a multimeter, as the dependent variable (Y-axis). Check the nature of curve obtained. Instead of a lemon, you can try other acidic foods like potatoes, citrus fruits like orange or lime to make the battery.
Electricity projects give you immediately observable results and the quantities involved can be easily measured. Hence, kids will learn a lot in a fun way.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
July 23, 2015
Generate Electricity with a Lemon Battery
A tingly science project from Science Buddies
By Science Buddies
Did you know you can make a battery out of a piece of fruit? You'll be charged up on science when you feel the success of your homemade electricity!
George Retseck
Key concepts Electricity Batteries Electrochemical reaction Electric conductor
Introduction Can you imagine how your life would change if batteries did not exist? If it were not for this handy way to store electrical energy, we would not be able to have all of our portable electronic devices, such as phones, tablets and laptop computers. So many other items—from remote-control cars to flashlights to hearing aids—would also need to be plugged into a wall outlet in order to function.
In 1800 Alessandro Volta invented the first battery, and scientists have been hard at work ever since improving previous designs. With all this work put into batteries and all the frustration you might have had coping with dead ones, it might surprise you that you can easily make one out of household materials. Try this activity and it might just charge your imagination!
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Background Batteries are containers that store chemical energy, which can be converted to electrical energy—or what we call electricity . They depend on an electrochemical reaction to do this. The reaction typically occurs between two pieces of metal, called electrodes , and a liquid or paste, called an electrolyte . For a battery to work well, the electrodes must be made up of two different types of materials. This ensures one will react differently than the other with the electrolyte. This difference is what generates electricity. Connect the two electrodes with a material that can transport electricity well (called a conductor ) and the chemical reactions fire up; the battery is generating electricity! As you make connections, note that electricity likes to take the path of least resistance. If there are multiple ways to go from one electrode to the other, the electricity will take the path that lets it flow most easily.
Now that you know the essentials of a battery, let's examine some household materials. Aluminum foil is a good conductor—electricity flows easily through it. The human body conducts electricity as well, but not as well as aluminum foil. Electrodes are as common as copper pennies you might have stashed in your piggy bank. As for electrolytes, they are found all over the kitchen; lemon juice is just one example. A simple household battery might be easier to make than you imagined!
At least two pennies
A few drops of dishwashing soap
Paper towels
Aluminum foil (at least nine by 60 centimeters)
At least one lemon (preferably with a thin skin)
Knife (and an adult's help when using it)
At least two plastic-coated paper clips
Preparation
Wash your pennies in soapy water, then rinse and dry them off with a paper towel. This will remove any dirt sticking to them.
Carefully cut three aluminum foil rectangles, each three centimeters by 20 centimeters.
Fold each strip in thirds lengthwise to get three sturdy one-centimeter-by-20-centimeter aluminum strips.
Note: In this activity you will make a very low-voltage battery. The amount of electricity generated by this homemade battery is safe, and you will even be able to test it by touching your finger to it and feeling the weak current. Higher voltages of electricity, however, can be very dangerous and even deadly; you should not experiment with commercial batteries or wall outlets.
Place the lemon on its side on a plate and have an adult carefully use the knife to make a small cut near the middle of the lemon (away from either end). Make the cut about two centimeters long and one centimeter deep.
Make a second, similar cut about one centimeter away and parallel to the first cut.
Push a penny in the first cut until only half of it is showing above the lemon skin. Part of the penny should be in contact with the lemon juice because that is what serves as the electrolyte. This copper penny in contact with the lemon juice serves as your first electrode. Note: If your lemon has a very thick skin, you might need an adult to carefully cut away some lemon peel. Why do you think is it important for part of the penny to be in contact with the lemon juice?
Slide one of the aluminum strips in the second cut until you are sure part of the aluminum is in contact with the lemon juice. Can you guess which part of a battery the aluminum strip that sits inside the lemon is? Do you think it is important for the aluminum to be in contact with the lemon juice?
You have just made a battery! It has two electrodes made of different metals and an electrolyte separating them . Do you think this battery is generating electricity or is there still something missing?
Your battery can generate electricity but will only do so when the electrodes are connected with something that conducts electricity. To make a connection attach the second aluminum strip to the part of the penny sticking out of the lemon with a plastic-coated paper clip. Make sure the aluminum touches the penny so electricity can pass between the copper and aluminum. You used an aluminum strip to create a connection; would you expect a plastic strip to work as well? Do you know why you do not need to create a connection to the second electrode for this particular battery?
As soon as the two aluminum strips touch one another, electricity will be produced in the battery and flow through the strips, from one electrode to the other. Because you cannot see the electricity flowing, you can try to feel it. Keep the two strips about one centimeter apart and touch your fingertip to them. Can you feel a tingling, created by a small amount of electricity running from one aluminum strip to the other through your body ?
For more electrical juice (and slightly stronger tingling sensation), you can build a second battery, identical to the first. You can choose a different spot on the lemon you just used or use a second lemon to build a second battery. Note that you only need one aluminum strip to build a second battery. To connect the second one to the original find the aluminum strip of the first battery that serves as electrode. (It has its end inserted in the lemon.) Use a plastic-coated paper clip to attach the other end of this aluminum strip to the penny of the second battery. This connects the aluminum electrode of the first battery to the copper electrode of the second battery.
Test this set of connected batteries in a similar way as you tested the single battery, bringing the ends of the two aluminum foil strips sticking out of your battery set (those that have a free end) in contact with your fingertip. Can you feel electricity running? If you could feel it well the first time, is this any different? (Note: If you cannot feel the tingling sensation, check if each electrode—pennies and the aluminum strips stuck in the lemon—are inserted deep enough so they are in contact with lemon juice; make sure there is firm contact between the penny and its attached aluminum strip; and that the aluminum strips are not touching one another. If all is correct, maybe you need slightly more electricity to feel tingling. You can test another person to see if he or she can feel the electricity or you can opt to add one more lemon battery to your set.)
Extra: Now that you can detect whether electricity is generated or not, try some different configurations. What happens if you let the aluminum strips touch? What happens if you replace an aluminum strip with a plastic piece, an unfolded metal paper clip or a toothpick?
Extra: Scientists call the way you connected your batteries in this activity "connecting batteries in series." Do you think the way you connect two batteries makes a difference in the amount of electricity you felt? Try it out by connecting the two copper electrodes to one another and attaching the two aluminum electrodes in the same way. (Note: You will need an extra strip of aluminum to do this.) Scientists call this "connecting batteries in parallel." Test both ways of connecting batteries and compare. Do you feel a difference?
Extra: Try different types of metals as electrodes for your batteries. Do you think a battery with two pennies as electrodes would generate electricity? What about a battery with a penny and a nickel ? Note that some combinations might generate electricity but the amount generated might be below your ability to feel it. Connecting two or more of these batteries might help you identify good combinations.
Extra: You used a lemon to provide the electrolyte for your battery. Do you think other vegetables or fruits would work as well? Would a potato, apple or onion battery work? Try a few from around the kitchen (with permission, of course). Does one particular fruit or vegetable outperform the others? With what you learned about how batteries generate electricity, why do you think that one type of produce made a stronger battery?
Extra : If you have an LED (light-emitting diode) available, investigate how many lemon batteries are needed to light it.
[break] Observations and results Did you feel the tingling in your fingertip?
The battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. It will generate electricity as soon as the electricity has a path to flow from one electrode to the other. You created this path using strips of aluminum, a material that conducts electricity well.
By connecting your battery to your fingertip, you allowed the small amount of electricity it generates to run through your body. This amount of electricity can create a tingling feeling in a fingertip. Experiences will differ from person to person. Some people might only feel the bigger signal generated by connecting several batteries in a particular way. Letting the aluminum strips touch provides a very easy way for the electricity to run from one electrode to the other, so almost no electricity will travel through your body and the tingling sensation disappears. Plastic and wood do not conduct electricity well; none will be felt when using these materials as connections. Metals, on the other hand, conduct electricity well. Different combinations of metals as electrodes will influence the amount of electricity generated. Using identical metals as electrodes will not generate electricity, however.
In this activity you made a very low-voltage homemade battery. But using commercial batteries can be dangerous—and never experiment with wall outlets!
More to explore Batteries , from ExplainThatStuff! How Do Batteries Work? , from LiveScience A Battery That Makes Cents , from Science Buddies Potato Batteries: How to Turn Produce into Veggie Power! , from Science Buddies
This activity brought to you in partnership with Science Buddies

Discover Explore Learn
How to Make Lemon Battery Science Fair Project – Harnessing the Energy
Have you ever wondered if you can power a light bulb with a lemon? Or how a lemon can act as a battery?
In this blog post, we will show you how to make a lemon battery science fair project that can harness the energy from a lemon and use it to light up an LED. This is a fun and easy experiment that you can do at home or in the classroom, and learn about the concepts of electricity, chemical reactions, and circuits.
Let’s get started!
What You Need

To make a lemon battery, you will need the following materials:
- A lemon (or any other citrus fruit)
- A zinc nail (or a galvanized nail )
- A copper coin (or a piece of copper wire)
- An LED (or a small light bulb)
- Alligator clips (or wires)
- A voltmeter (optional)
How It Works
A lemon battery is a type of electrochemical cell , which is a device that converts chemical energy into electrical energy. An electrochemical cell consists of two electrodes (the zinc nail and the copper coin) and an electrolyte (the lemon juice).
Electrochemical Processes
The electrodes function as conduits for electricity, while the electrolyte facilitates the flow of electric charge. When these components are inserted into the lemon, a chemical reaction ensues, primarily involving the zinc and the lemon juice.
As the zinc dissolves in the lemon juice, it releases negatively charged electrons. These electrons then traverse from the zinc nail to the copper coin through the external circuit, which includes components like the LED and wires.
This movement of electrons constitutes an electric current, providing the power necessary to illuminate the LED.
Unique Reactions and Limitations
Simultaneously, the copper coin undergoes a distinct reaction with the lemon juice. It accepts electrons from the lemon juice, leading to a positive charge.
The lemon juice, containing positively charged hydrogen ions, is attracted to the negatively charged copper coin. The combination of hydrogen ions and electrons results in the formation of hydrogen gas , which escapes as bubbles from the lemon.
The functionality of the lemon battery relies on a sufficient supply of zinc and lemon juice to sustain the ongoing chemical reaction. Unfortunately, as time progresses, the zinc and lemon juice are depleted, causing a reduction in electric current.
Eventually, the lemon battery exhausts its resources, resulting in a loss of power and the cessation of the LED’s operation.
How to Make It

Create a lemon battery with the following steps:
- Begin by cutting the lemon in half and giving it a slight squeeze to release some juice.
- Insert the zinc nail into one lemon half, ensuring it avoids contact with the peel.
- In the other lemon half, insert the copper coin, making sure it remains peel-free.
- Connect one end of an alligator clip to the zinc nail and the other end to the longer leg of the LED; this marks the positive terminal.
- Attach another alligator clip to the copper coin and the opposite end to the shorter LED leg; this signifies the negative terminal.
- Observe the LED to check for illumination. If it doesn’t light up, experiment by reversing the connections or trying a different LED.
How to Measure It
When evaluating the power of your lemon battery, the measurement of voltage and current is crucial. Utilize a voltmeter for voltage and an ammeter for current.
Alternatively, a multimeter , capable of measuring both, can streamline the process.
Voltage Measurement
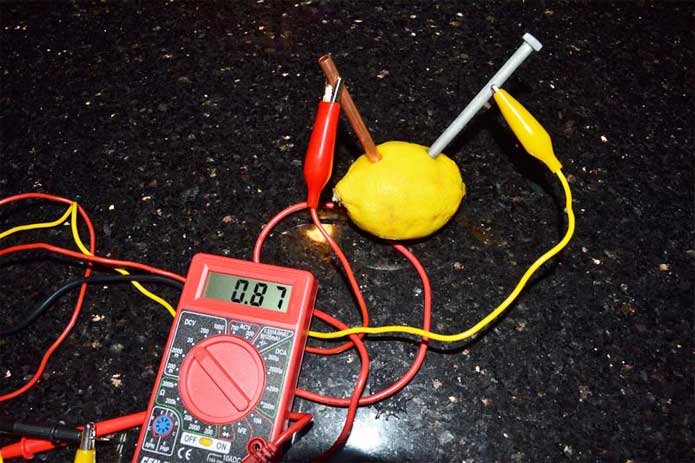
In order to check the power of your lemon battery, use a voltmeter for voltage and an ammeter for current. A voltmeter measures the electric potential difference , while an ammeter measures the current in the circuit.
You can also use a multimeter, which is a device that can measure both voltage and current. To measure the voltage of the lemon battery, follow these steps:
- Set the voltmeter (or the multimeter) to the DC voltage mode, and choose a suitable range (for example, 2 volts).
- Connect the positive probe of the voltmeter to the positive terminal of the lemon battery, and the negative probe to the negative terminal.
- Read the voltage on the display of the voltmeter. It should be around 0.9 volts, depending on the size and freshness of the lemon.
Current Measurement
To measure the current of the lemon battery, follow these steps:
- Set the ammeter (or the multimeter) to the DC current mode, and choose a suitable range (for example, 10 milliamps).
- Disconnect the alligator clip that connects the LED to the negative terminal of the lemon battery, and insert the ammeter in between. This means that the current will flow from the lemon battery to the LED, to the ammeter, and back to the lemon battery.
- Read the current on the display of the ammeter. It should be around 1 milliamp, depending on the brightness of the LED.
How to Improve It
To make your lemon battery more powerful, you can try the following ideas.
Use More Lemons
You can connect several lemons in a series, which means that you connect the positive terminal of one lemon to the negative terminal of another lemon, and so on. This will increase the voltage of the lemon battery, and make the LED brighter.
You can also connect several lemons in parallel, which means that you connect the positive terminals of all the lemons together, and the negative terminals of all the lemons together. This will increase the current of the lemon battery, and make the LED last longer.
Experiment with Various Electrodes
Explore the impact of different metals like iron, aluminum, or silver on the voltage and current of your lemon battery. Vary the shapes and sizes of electrodes, such as nails, screws, or wires, to observe how they influence the surface area and resistance of the lemon battery.
Explore Different Electrolytes
Test the effects of using various liquids, such as orange juice, vinegar, or saltwater, on the acidity and conductivity of the lemon battery. Additionally, experiment with different substances like baking soda, sugar, or bleach to observe how they influence the chemical reaction and overall performance of the lemon battery.
How to Explain It
To explain your lemon battery science fair project, you can use the following table to summarize your findings:
| Variable | Effect | Explanation |
|---|---|---|
| Number of lemons | Increased lemons in series boost voltage, while more lemons in parallel enhance current. | Adding lemons in series accumulates electric potential difference. Adding lemons in parallel increases the available electric charge for flow. |
| Type of electrodes | Diverse metals exhibit varying reactivity and conductivity. | More reactive metals release more electrons, generating higher voltage. More conductive metals facilitate increased electron flow, creating a higher current. |
| Type of electrolyte | Various liquids vary in acidity and conductivity. | More acidic liquids yield more hydrogen ions, leading to higher voltage. More conductive liquids enhance electric charge flow, resulting in higher current. |
You can also use the following diagram to illustrate how the lemon battery works:
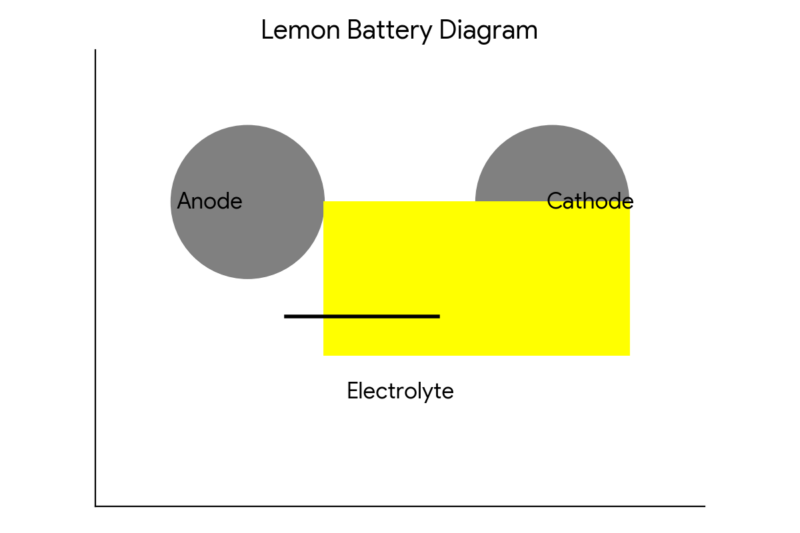
In this diagram, the zinc nail is the anode, which is the electrode where oxidation (loss of electrons) occurs. The copper coin is the cathode, which is the electrode where reduction (gain of electrons) occurs.
The lemon juice is the electrolyte , which is the medium that allows the electric charge to flow. The LED is the load, which is the device that uses electric energy.
You can also use the following equation to describe the chemical reaction that occurs in the lemon battery:
Zn(s) + 2H⁺(aq) → Zn²⁺(aq) + H₂(g) + 2e⁻
In this equation, the zinc atom loses two electrons and becomes a zinc ion, which dissolves in the lemon juice. The hydrogen ion gains two electrons and becomes a hydrogen atom, which forms hydrogen gas.
The electrons flow from the zinc nail to the copper coin through the external circuit.
How many lemons do I need to light up an LED?
For effective LED illumination, use multiple lemons based on the LED’s type and size. Some LEDs require more voltage or current than a single lemon can deliver. Enhance the lemon battery by connecting two or more lemons in series or parallel. Experiment with larger or fresher lemons to optimize overall performance.
What are some real-life applications of lemon batteries or similar devices?
Lemon batteries serve as emergency power sources, educational tools, and environmental sensors. They power devices like clocks, radios, and calculators, aiding educators in teaching electricity and chemistry principles. Researchers use lemon batteries to measure soil or water pH levels.
Why do I need to use metals with different reactivity for the electrodes?
Choose metals with varying reactivity for electrodes to establish an electric potential difference. More reactive metals become anodes, losing electrons easily, while less reactive metals become cathodes, adept at gaining electrons. A greater reactivity difference results in a higher lemon battery voltage.
How can I test the pH level of the lemon juice or other liquids?
Assess the pH level of liquids using pH indicators like litmus paper, phenolphthalein, or red cabbage juice. Observe color changes in the indicator when dipped into the liquid, comparing them to a pH scale ranging from 0 to 14, where lower values indicate acidity, higher values denote alkalinity, and 7 represents neutrality.
What are some safety precautions to take when making a lemon battery?
Prioritize safety by wearing gloves, goggles, and aprons when crafting a lemon battery to protect against lemon juice and sharp metal edges. Minimize injury risk by avoiding direct contact with electrodes and wires. Dispose of the lemon battery responsibly to prevent leakage or corrosion.
How can I make a lemon-battery clock or a lemon-battery calculator?
Utilize the materials and steps from the lemon battery LED process to craft a lemon battery clock or calculator. Substitute the LED with the desired device and connect the lemon battery’s positive and negative terminals to the corresponding clock or calculator terminals. Adjust the lemon quantity or electrodes as needed to meet the power requirements of the specific device.
What are some other fruits or vegetables that can be used to make a battery?
Explore battery options with various fruits or vegetables such as potatoes, tomatoes, apples, oranges, grapefruits, limes, cucumbers, carrots, or onions. These items contain acids or salts acting as electrolytes, akin to lemon juice. Keep in mind that the battery’s voltage and current may fluctuate based on the type and freshness of the chosen fruit or vegetable.
Final Words
In this blog post, we have learned how to make a lemon battery science fair project that can harness the energy from a lemon and use it to light up an LED. We have also learned how to measure, improve, and explain the lemon battery.
We hope that you have enjoyed this experiment and learned something new. Have fun and stay curious!
Related Posts:
- How to Make a Volcano for a Science Fair Project -…
- Science Fair Project Idea: Why Do Some Foods Mold…
- 101 Science Fair Ideas for 8th Graders: Unleash Your…
- Balloon Rockets: A Journey Into Science and Fun
- 7 Best Youtube Science Channels for Kids: Turn…
All Science Fair Projects
1000 science fair projects with complete instructions, electricity from a lemon.

Method & Materials
Why do this project, also consider, full project details, related videos.
Related Science Fair Project Ideas
Lemon and Potato Battery Experiment
Learn how to generate electricity from common fruit or vegetables.
Posted by Admin / in Energy & Electricity Experiments
Is it possible to produce electricity from common fruit or vegetables? Fruits and vegetables require energy from the sun to grow and produce a harvest. Is it possible that some of the sun's energy is stored in the produce for our use? We know that by eating fruits and vegetables our body can convert this food to energy. Is it possible to directly generate electricity from a piece of fruit or a vegetable. This lemon battery and potato battery science experiment tests this theory.
Materials Needed
- Copper strip or rod
- Zinc strip or zinc-coated bolt
- Circuit wire or alligator clips with wire
EXPERIMENT STEPS

Step 1: Cut 2 small slits in the skin of both the lemon and the potato. Make the slits are a few inches apart.

Step 2: Push the copper and zinc strips into the slits in each piece of produce. Make sure the rods do not touch each other.

Step 3: Connect an electrical wire to the end of each metal strip. Alligator clips make this step easy.

Step 4: Measure the voltage drop between the two wires attached to the metal strips on the lemon and the potato. This is the amount of voltage being produced by each piece of produce. Compare the difference in the amount of voltage produced by a lemon and a potato. What do you notice? How long will the fruit and vegetable generate voltage?
Science Learned
The lemon and the potato act like a low-power battery. This experiment shows how a wet cell battery works. Chemicals in the fruit or vegetable create a negative charge in the zinc strip. Electrons move into the zinc strip and travel up the wire attached. The electrons then travel through the voltmeter which measures the voltage drop and end up in the copper strip which becomes the positive end of the circuit. Pardon the pun, but from this experiment we can say that it is possible to "produce electricity".
Oberlin College: Demonstration of lemon battery powering a buzzer .
U.S. Dept. of Energy: Calculating Lemon Battery Power Q&A
- About the author
- Back to Experiment
Please select the social network you want to share this page with:
We like you too :)
Thanks for taking time to give us feedback!
- Energy & Electricity experiments
- science experiments for kids
- lemon and potato battery experiment
- lemon battery experiment
- fruit battery
- potato battery experiment/a>
- vegetable battery

posted by Admin
- previous experiment
- next experiment

How to Make a Simple Battery
in Energy and Electricity Experiments
Make a simple battery using coins and other common items.

Solar Energy Experiment for Kids
Teach kids how light is used to generate electricity in this solar energy experiment.

Beginner Electronics Experiment For Kids
This experiment is a good starting point for kids to begin learning about electronics.

LED Solar Circuit Experiment
Learn how to make an electrical circuit to power an LED using solar power.

How to Make an Electromagnet
Test the relationship between electricity and magnetism by making an electromagnet.

Power a Light with Static Electricity
Use static electricity to power a light bulb!
- For Teachers

- Everyday Activities
- Experiments

Lemon Batteries
Can you get power from a lemon?
Batteries consist of two different metals suspended in an acidic solution.
Is it possible to use the acid in a lemon to power a light? Try it to find out!
Watch the video on YouTube: QYZE-SrpoJ4
You Will Need
4 or more large, fresh, juicy lemons or other citrus fruits
A kitchen knife
4 or more zinc electrodes You can find galvanized washers or roofing nails at most hardware stores, or you can purchase either zinc or magnesium wire online
4 copper electrodes Copper-coated pennies work, or you can find bare copper wire or copper plumbing fittings at most hardware stores
1 light-emitting diode (LED) component We recommend a red LED because they typically need lower voltages to glow than other colors, but many colors will work. The best LEDs for this experiment are designed to glow with a low current such as this set from Amazon , or you can purchase a lesser quantity from Mouser electronics .
6 or more lead wires with alligator clips. Such as this set available on Amazon
Multimeter (optional)
Materials & Directions PDF
NOTE: Some retailers also sell lemon battery kits that can include a buzzer or a low voltage clock. These instructions assume you are using your lemon battery to make an LED glow.
- Ask your scientist to create a testable question.
- Carefully clean your zinc and copper electrodes to remove any dirt or grease. (Careful not to scrub all of the zinc coating off the galvanized washers or nails.)
- Roll one lemon on a hard surface while pushing down to break the cell walls and loosen up the juice inside. The sour (acidic) juice is needed for the chemical reaction that you are about to start.
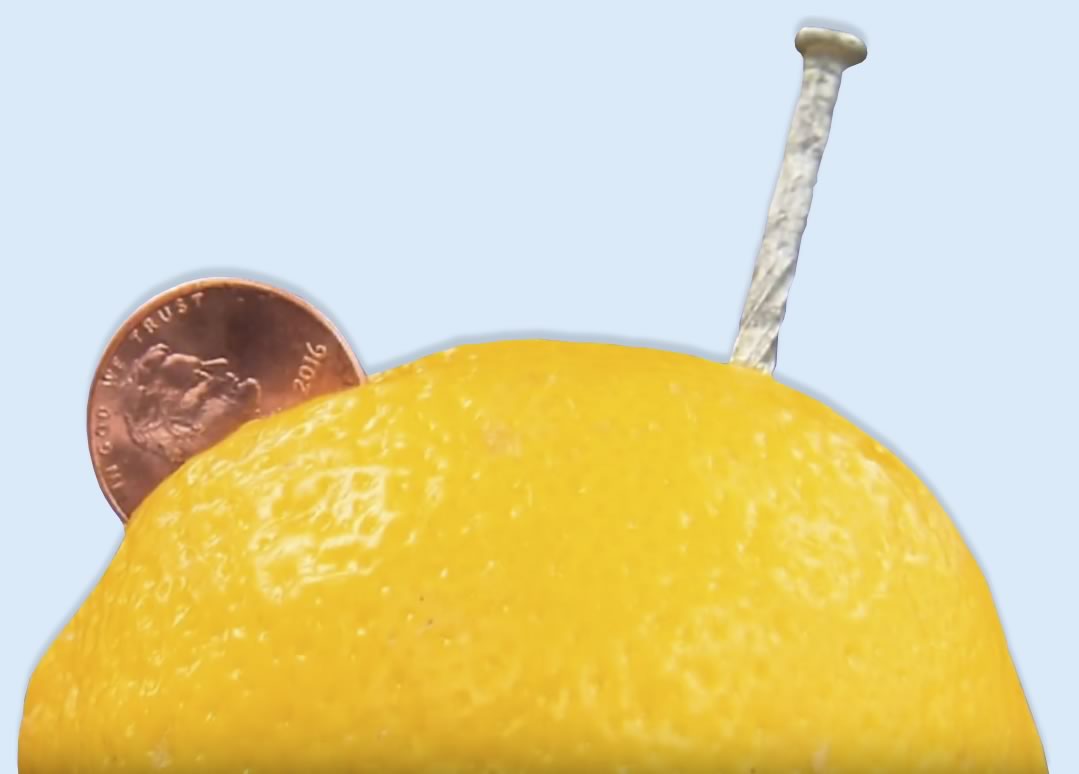
- Place the lemon on its side on a plate and have an adult carefully use the kitchen knife to make a 2 small cuts in the top of the lemon. Make each cut about two centimeters long, one centimeter deep, and about 0.5-1 centimeters apart. (To conserve lemons, you can cut 1 lemon in half, and put the electrodes in either the cut side or the rind side.)
- Insert one zinc electrode deep into one of the cuts, and one copper electrode deep into the other. Leave some sticking out so you can connect your wires to them. You have now made a lemon cell!
- Set the multimeter to measure DC voltage (V with a straight line). If it has scale options, set it to measure 2000 millivolts (2000m).
- Hook two alligator clips from your leads to the two electrodes. Attach red lead of the multimeter (+) to the copper electrode, and the black lead of the multimeter (-) to the zinc electrode.
- Measure the voltage from your lemon cell. The average lemon cell should read about 0.9–1.0 volts.
- Now set the multimeter to measure current (A with a squiggly line: mÃ/Ã). If it has scale options, set it to measure 1–20mÃ.
- Measure the current of your lemon cell. It should read a few tenths of a milliampere. Some multimeters are not sensitive enough to measure currents less than one milliampere, in which case you will see 0.0 as the reading.
- A red LED typically needs a voltage of 1.2–1.6 V, so we need more power to light the bulb.
- Follow steps 3-5 to make 3 or 4 more lemon cells.
- (Optional) If you have a multimeter, check each lemon battery to make sure it generates voltage and current.
- Connect the zinc electrode on the first lemon to the copper electrode on the second lemon.
- Connect the zinc electrode on the second lemon to the copper electrode on the third lemon.
- Repeat if using more lemons. This type of connection is called a series circuit , and provides one path through which electricity can flow.
- Gently bend the lead wires of the LED apart from each other.
- Connect a lead wire from the copper electrode of the first lemon cell to the longer lead wire from the LED.
- Connect a lead wire from the zinc electrode of the third cell to the shorter wire of the LED.
- Turn down the room lights to see if your LED is glowing! If it isn’t, see the troubleshooting tips below.
Troubleshooting your lemon battery:
- Ensure the electrodes are not touching inside lemon.
- Ensure the alligator clips on the test lead wires are not touching each other where you connect them to the LED.
- The wires from one lemon to the other have to be connected from zinc to copper in order for the electricity to flow.
- Is it an old lemon? The lemon needs to be juicy inside.
- Do you need to add more lemons?
- Is your LED broken? Or does it require a higher voltage to work?
- The quality of the copper and zinc can be problematic. Pennies are rarely pure copper. Try substituting a length of 14 gauge copper wire (common house wire). Experiment with different lengths and configurations of electrodes. Other sources of zinc and copper may be found in the plumbing department of a hardware store.
Discovery Questions
Beginning the experiment, during the experiment, after the experiment, how it works.
Electrochemical cells, also called batteries, require three things—two electrodes and one electrolyte. One of the electrodes has to have a stronger desire for electrons than the other—in chemistry we say that it has a higher electronegativity . The electrode that wants the electrons more is called the cathode , and the one that gives up electrons is electropositive and is called the anode .
Copper likes having electrons more than zinc so it’s more electronegative and is the cathode, leaving zinc to be the anode. An electrolyte is a solution that conducts electricity. The lemon provides citric acid, and acids contain ions which conduct electricity, making the lemon our electrolyte .
When zinc is exposed to the acid in the lemon juice, the acid oxidizes—or removes electrons from the zinc. The resulting positively charged zinc ions move into the lemon juice, and the resulting electrons collect in the zinc metal. They then rush across the wire into the copper which wants electrons more than zinc. Those electrons, now in the copper, pull a couple of protons or hydrogen ions out of the acid and reduce them, adding electrons, which creates hydrogen gas. If we could see inside the lemon, we might be able to see very very tiny bubbles of hydrogen gas forming on the copper electrode. In summary, the electricity is not coming from the lemon by itself, but from the chemical reaction resulting from the differences in electronegativities between zinc and copper.
Source: Dr. Christopher Gorski and Jenelle Fortunato, Penn State College of Engineering
Babble Dabble Do
How to Make a Lemon Battery and a Lime Light
February 5, 2020 by Ana Dziengel 1 Comment
One of the most classic science fair projects you can do is learn how to make a lemon battery. In fact along with volcanoes this is one of the standards I see at the science fair each year and for good reason, it’s incredible to use chemistry to generate an electric current! Today I’m going to show you how to make a lemon battery and it’s cousin a “Lime Light.” I’ll also walk you through how to turn this classic project into a science fair experiment!
This post contains affiliate links.
This project is featured in my book STEAM Play & Learn ! It’s a wonderful project for parents and kids to do together. To see my book and more easy STEAM project for preschool aged kids and up hop over here:
How to Make a Lemon Battery
Lemon battery/lime light materials.
- (6-7) Lemons/Limes
- Galvanized nails (Find these at the hardware store)
- Copper Wire
- Wire Cutters
- Electrical Tape
- Alligator Clips (optional but recommended)
Lemon Battery & Lime Light Instructions
Time needed: 20 minutes.
Learn how to make a lemon battery. Limes may be used interchangeably in this project. I like to call it a Lime Light!
Cut a 2” length of copper wire.
Stick the end of the wire into one side of the lemon.
Place a galvanized nail in the lemon/lime approximately one away inch from the wire.
If you have a volt meter, connect the red test lead to the nail and the black lead to the copper wire with binder clips. You should see a reading of almost 1 volt.
To make enough power to turn on a 6V LED you will need to make more batteries.* To make a series of (6) connected lemon batteries cut (6) roughly 3”-4” long pieces of copper wire. Wrap each piece of wire around a galvanized nail. Leave 2” of the nail unwrapped.
Place one copper wrapped galvanized nail in the first lemon/lime. Place a 2″ piece of copper wire in the other end of the lemon/lime. If you are reusing your single battery from steps 1-3, remove the galvanized nail and replace it with a copper wrapped nail. Keep the unconnected piece of copper wire on one side of the lemon/lime.
Place the unwrapped end of the copper wire around the nail in a second lemon/lime. Be sure to press it into the lemon flesh.
Add a copper wrapped nail into the second lemon/lime and place its free copper end into a third lemon. Repeat until you have 6-7 lemons/limes in a series. Make sure they are placed in a circular shape. The seventh lemon/lime should end with an unwrapped nail in it.
Take your LED and spread the leads apart. Connect one end of an alligator clip to the long (positive) leg of the LED and the other end to the copper wire in the first lemon/lime. Connect a second alligator clip to the shorter (negative) leg of the LED and the other side to the galvanized nail in the last lemon/lime. The LED should turn on! If you don’t have alligator clips use electrical tape to secure the long (positive) leg of the LED to the copper wire in the first lemon/lime and the shorter (negative) leg of the LED to the galvanized nail in the seventh lemon/lime.
Troubleshooting
- If you have a voltmeter, test the circuit to see where the power is stopping. Usually a connection is reversed or not fully inserted into the lemon.
- If the LED does not turn on reverse the LED legs. Be sure to check the connections in each lemon/ lime to make sure they are secure.
How big can you go?
At our Camp STEAM we attempted to make HUGE citrus battery by combining each of our camp team’s 6 cell battery together. We were able to get a read of 24 volts on our voltmeter with this battery! Unfortunately we also discovered it would not power a 12 V alarm and were not sure why.
One of our favorite science channels on YOuTube also tried to make GIANT lemon battery with hundreds of lemons but it also did not work as expected. Check out Mark Rober’s video here.
Let’s Talk STEAM
The Science: Batteries have three parts, a positive side called a cathode , a negative side called an anode , and a material in between called an electrolyte . A chemical reaction occurs in batteries causing electricity to build up in the anode until it forced to flow out of the battery in one direction.
In our lemon/lime battery, the nail acts as the cathode, the copper acts as the anode and the lime is the electrolyte. The two different types of metals, a zinc nail and a copper wire cause a chemical reaction that creates a flow of electricity.
The Technology: Our lemon/lime batteries each only make 1 volt of power but when you connect 6-7 of them together you can generate the 6 volts of electricity. This is a simple use of a chemical reaction to create enough power to turn on a light.
Turn it into a science fair project
Let’s turn this topic into an actual experiment! Here’s how you can take this to the science fair:
- Ask yourself questions How can I increase the power I get from each lemon battery? What happens if I add more nails and more wire? Can I power a motor or small alarm with a lemon battery? Do other citrus fruit work in this experiment? Do other fruits or tomatoes work? How to regular batteries work and how are they similar and different to a fruit battery?
- Research Do research online and at the library to try and predict the answer to your question. Read about how voltage is calculated. Read about the chemical reaction occurring within each lemon battery to understand the type of power it is generating.Read about the metals zinc and copper to understand why they react with citric acid. Research other fruits or vegetables that might also contain citric acid.
- Make a hypothesis A hypothesis is your prediction of the answer to your question based on your research. It may or may not be true.
- Experiment! Test your hypothesis by testing the variables and documenting them. Be sure to take notes of each experiment and what happens; this is called your data.
- Draw a conclusion Based on your experiments form a conclusion. Was your hypothesis correct?
- Share your findings Create a presentation with your findings. Include your research, hypothesis, the data you collected, and your conclusions. Be sure to include images and samples!
Experiment Examples:
Try other fruits and vegetables Experiment with other citrus fruits to see how much power they generate. Try other acidic vegetables and fruits such as tomatoes, apples, and potatoes. Document which produce the most and least or no current. Try making a large battery from a series of different fruits and vegetables.
Try making lemon batteries with multiple nails and wires. Does putting two or more nails and copper wires in one lemon have an effect on the voltage?
What else can you power? Besides powering an LED can you make a small motor move or a small alarm go off? If not why?
If you enjoyed this electronics project for kids here are some more ideas:
Are you passionate about raising creative kids?
Join over 22,179 parents and educators who want connect with kids and nurture their creative process through magical, easy projects you can do TOGETHER.
Subscribe to our email list to receive project ideas as well as offers for some our creative products.
If you want to read our privacy policy before subscribing, hop over here. Get Babble Dabble Do delivered straight to your inbox!
February 7, 2020 at 8:27 pm
Awesome post– I love this classic battery demonstration, and what a fun challenge to connect the lemon batteries together to drive up the voltage!
24 volts is impressive, but the current generated by lemon batteries is very low– that’s probably why the big lemon battery wouldn’t power the motor.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Pin It on Pinterest
Choose an Account to Log In

Notifications
Science project, how to make a lemon battery.

Has your flashlight ever stopped working because the batteries were dead? It’s no fun walking around in complete darkness. Batteries are everywhere—in our toys, in our cars, in our flashlights and cell phones. But how do they work? What makes them stop working? You can learn how to make a lemon battery to learn more about these very important devices.
How does a battery work?
- A lemon, or other citrus fruit
- 18 (or smaller) gauge copper wire
- Wire stripper/clipper
- A grown-up or older friend
- Steel paper clip, small galvanized nail (one that is covered in zinc), or a piece of zinc (ideal)
- Ask your grown-up to use the wire strippers to first strip about 2 1/2 inches of plastic insulation off the copper wire. Then, request that the grown-up clip that piece of stripped wire off of the main roll.
- Carefully straighten the steel paper clip. Use the wire clippers to cut it to the same length as your copper wire.
- Use the sandpaper to rub out any rough spots in your wire or paperclip. You are going to be touching the wire ends to your tongue, so you want them to be smooth. If you are using the zinc covered nail or piece, scratch it lightly with the sand paper to expose a fresh surface.
- Roll the lemon gently on a table to break the cell walls and loosen up the juice inside. The sour juice is needed for the chemical reaction that you are about to start. The fact that the juice is sour should give us some hints about what kind of chemicals make up lemon juice. What do you think the sour flavor might tell us?
- Carefully stick the copper wire about 1 inch into the lemon.
- Make sure your tongue is moist with saliva , or spit. Touch your tongue to the copper wire. Do you notice anything?
- Stick the paperclip, zinc covered nail or zinc strip into a spot in the lemon about 1/4 inch away from the copper wire. Make sure the wires don’t touch. The wires need to be close to each other because they will be swapping matter in the chemical reaction. If they are too far apart, the matter might lose their way.

- This time, touch your moistened tongue to both wire ends. What do you notice?
When you touched your tongue to just the copper wire, you most likely would not have noticed anything unusual. When you touched your tongue to BOTH of the metal ends, you might have felt a tingle, or noticed a metallic taste.
The tingle or metal taste you noticed shows that your lemon battery was generating an electric current . That means tiny electrons were moving across the surface of your tongue. Electrons are subatomic particles that zoom around an atom’s center and make up the part of the atom that is negatively charged.
The lemon battery you made is a type of battery called a voltaic battery . These types of batteries are made of two different metals, which act as electrodes , or places where electrons can enter or leave a battery. In your case, the electrical current entered your tongue, which is why you felt a tingle.
So why were we able to stick electrodes into a lemon and get a battery? All voltaic batteries need their metals to be placed in an electrolyte . An electrolyte is a substance that can carry electrical current when dissolved in water. The tiny bit of salt in your saliva makes your saliva an electrolyte, and the sour citric acid does the same thing for lemon juice. Batteries stop working when there is not enough of the electrolyte to react with the metal or not enough metal left to react with the electrolyte.
Going Further
You can generate more electrical current by connecting multiple lemon batteries. Just make a second battery and connect the zinc or steel piece of one battery with the copper wire of the other battery using another piece of copper wire to act as a bridge.
You can use your enlarged lemon battery to power a low-power device like a digital watch or calculator. Remove the regular battery from the digital watch or calculator. Then, hook up the copper electrode of your lemon battery with battery slot’s positive contact. Connect the zinc or iron electrode with the negative contact. Can you get the device to work?
If you are looking to test a variable, try making batteries using different fruits and vegetables. Which ones produce the biggest tingle on your tongue? Which ones generate the most electric current?
Related learning resources
Add to collection, create new collection, new collection, new collection>, sign up to start collecting.
Bookmark this to easily find it later. Then send your curated collection to your children, or put together your own custom lesson plan.
Fun Lemon Battery Science Fair Experiment for Kids
We all heard of making lemonade out of lemons and that when things go wrong, if you know to Think in The Right Way then you can make something good come out of a sour situation. But how can you make a BATTERY out of Lemons?!
Most of us know what Lemons look like; A Lemon is a yellow, oval, thick skinned and fragrant citrus fruit said to originate from India and is commonly grown in the Middle East .
Lemons are a good source of Vitamin C, which is important to boost our immune system in order to fight sickness. Lemons are used in aromatherapy, but they are not just healthy for you, they are also used for culinary purposes; lemon juice, lemon cakes, lemon cookies, a tarty lemon pie, lemon ice cream and in food preparations like Tahini, Hummus, salads and many other dishes in many countries around the world.
Lemons can also provide a lot of fun for your kids, even if they hate drinking lemon juice. One of the interesting things you can do with your kids and a few lemons, is a fun lemon battery science fair experiment ! It is an easy, low cost and fun educational activity to do at home or at school. Try this fun lemon science experiment with your kids to teach kids about electrical circuits!

How to Make a Battery from Lemons
What you need for the Lemon Battery Science Fair Experiment:
TIP: The power from one single lemon will not be strong enough to light a bulb or be used as a battery. You will need to connect several lemons together with metal wires for enough power or electricity to light a bulb from a lemon battery. Minimum two lemons will create a battery but 3 are better. See what happens if you add more lemons. What will the volt meter show? How strong will the bulb glow?
Fun Facts about Lemons
Lemon battery science fair experiment video for kids.

Lemon Light Experiment (How to Make a Lemon Battery)
- May 21, 2021
- 7-9 Year Olds , Physics
Performing STEM activities is the perfect approach to learning science for kids.
Today, we are going to perform such a classic STEM activity using ‘Lemon Fruit’. This experiment offers a great visual demonstration to children revealing the science behind a battery.

Using the ‘Lemon Light Experiment’, kids can create a direct current battery from Lemon. It is suitable for home-schoolers and elementary learners.
Making of Lemon Battery
Materials Required

1) 3 Fresh Lemons (The more lemons you use, the more power or electricity you can create)
2) LED bulbs
3) Three Copper strips or nails around 2 inches in length
4) Four sets of electric wires that have alligator clips
5) Zinc-coated Galvanized strips or nails about 2 inches in length
6) A sharp knife
Note: Adult supervision is compulsory if your children are performing this science activity on their own as we are dealing with electric circuits and knife.
Instructions to Perform Lemon Light Experiment
The lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries work. Using this activity, we cannot make a high voltage current. Let us learn how lemon fruit generates low voltage electricity.
Step-1: As a first step, pick three to four fresh lemons and place them side by side intact. And give two vertical cuts about half-inch in length vertically on the surface of each lemon using a sharp knife. Such that, for a total of three lemons you will give six incisions. Make sure you leave some space in between two incisions on each lemon to fix the electric wire.

Step-2: Now pick three galvanized nails and insert them into the three incisions alternatively out of six incisions on the three lemons.

Step-3: Later, pick the three copper nailsand insert them into the other three incisions on the three lemons. At this point, three lemons are fixed with three copper and galvanized nails alternatively.

Step-4: Now connect the electrical wires in the manner: to the one galvanic strip of one lemon to the copper strip of another lemon using alligator clips. Follow the same rule as the other electric wire sets to create a chain. But make sure the alligator clips present at the end sides of the end lemons are left free i.e. one copper and one galvanized strip are set free.

Step-5: As a final step, connect the free ends of alligator clips of electric wires on the end lemons to an LED bulb. Make sure you are connecting the first lemon alligator clip to the long part of the LED bulb while the last lemon alligator clip to the shorter part of the LED bulb.

As soon as you connect, you will see the LED bulb emitting light from inside. If you want a better demonstration, switch off the light in the room or make the room dark and observe the results.

Tips: If you fail to see the LED bulb lighting up, recheck all your connections. Make sure the LED bulb is also in the right position and connected rightly. You can change the direction of the bulb to see positive results.
How do Lemons produce electricity and make an LED bulb glow?
For batteries to work, we need three things 2 electrodes and 1 electrolyte. One of these electrodes should have the ability to attract electrons. The one which attracts more electrons is called cathode. The other electrode which gives up electrons is called anode.
In our experiment, the copper strip has more electrons so that will work as cathode. But when you connect a cathode with an anode – electrons cannot move freely. This is because Zinc also has protons which cannot move through the wires.
Thats where the acidic solution comes in to play – the lemon juice. Here, lemon juice acts as electrolyte. Electrolyte removes electrons from the zinc through an oxidation process. These positively charged ions from zinc moves in the acidic solution which then collected by the copper.
Once the electrons moves into the copper – they tend to pull couple of hydrogen ions or protons from the acidic solution to reduce them by adding electrons.
To summarize – you don’t get the electricity from the lemon but the electricity is generated based on the chemical reaction that happens between zinc, copper and acidic lemon solution.
The positively charged copper strip is more likely to attract the free electrons released from the galvanic strip. However, the movement of electrons inside the lemons creates an electric circuit and the circuit closes. As a result, the electric circuit gets closed by connecting alligator clips to the LED bulb.
In this closed circuit, the free electrons from the zinc galvanic strip move towards the copper strip through the LED bulb and make it light up.
Interesting Electricity Experiments for Kids:
Static Comb Experiment
How to Bend water with Static Electricity
Static Electricity Hair Experiment
Modifications you can do to the Lemon Light Experiment to extend the activity
Check out the extension ideas of the Lemon light experiment below and explore more science and principles of electric circuits.
1) Try the experiment using different types of citrus-based fruits and check whether they work effectively in producing electric current. If so, then calculate the amount of current produced by observing the intensity of the light-emitting inside the LED bulb.
2) You can also check the same experiment using fruits that are alkaline and find out why they don’t produce electric circuits.
3) Replace the conducting solution, lemon juice with other solutions like vegetable juices and even aqueous solution, and experiment. Check the results!
4) You can try using other kinds of metals as electrodes. Find the reasons and dissimilarities in the outcome.
5) Try out different electricity conducting materials and check the best combination of metals that make good electric voltage.
6) Change the connections you made in the experiment and observe the changes happening while experimenting.
Important questionnaire to discuss with the children
If you are a teacher or home-schooler, find out the list of hint questions that make your students and children active through the experiment and improve their observing skills.
1) Why does lemon juice acts as a good conducting solution?
2) What are other good conducting solutions among fruits and vegetables?
3) Explain what is electric current and circuits.
4) What role does shorter and longer ends of LED bulb play in the experiment?
5) Does the LED bulb light up when two identical metals utilized in the experiment as electrodes?
6) Compare water and salt concerning the conducting power of electricity and explore the differences in conducting electricity.
Leave a Reply Cancel Reply
Your email address will not be published. Required fields are marked *
Name *
Email *
Add Comment *
Save my name, email, and website in this browser for the next time I comment.
Post Comment
Lemon Battery
Activity length, chemical reactions electricity, activity type, discrepant event (investigatable).
You can make a battery using a piece of fruit?
Yes , technically, but not a very strong one!
The source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the lemon.
The citric acid of the lemon reacts with the zinc and loosens electrons. Copper pulls electrons more strongly than zinc, so loose electrons will move towards the copper when the electrodes are connected by wires. Moving electrons are called an electric current, which is what lights up the bulb.
Teacher Tip: This is a classic electricity activity, but it can be very frustrating if you don't have the right equipment. We recommend having a multimeter or voltmeter on hand to test the voltage.
Describe the relationship between an electron and current electricity.
Per Pair of Students: lemon (and other fruit, optional) 1 copper strip 1 zinc strip (you can use a galvanized nail, which is coated with zinc) knife 2 copper wire leads (each about 20 cm long) with alligator clips on both ends LED bulb with a rating of no more than 2 volts (the smaller the voltage, the better) wire cutters wire strippers
Per Class: multimeter or voltmeter (optional)
Key Questions
- What happens when you connect the wire to the bulb?
- What is the power source?
- What role does the lemon play in lighting up the bulb?
- When we use two strips of the same metal, does the bulb light up? Why?
- Is water a good conductor of electrical current? Is salt a good conductor of electrical current? How do you know?
- Roll the lemon firmly on a counter to release some of the juices.
- Insert the one copper strip and one zinc strip vertically into the lemon, with one end sticking out.
- Connect one wire lead to each metal strip (electrode).
- Connect one of the free ends of the wire leads to one of the wires attached to the LED
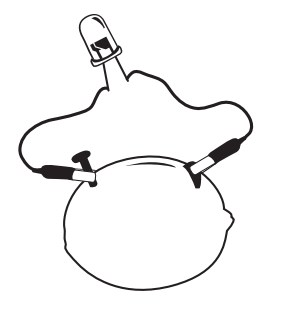
- Hint: If you’re using an LED, it will only light if it’s connected in the right direction. Try switching direction.
- Hint: Don’t try to test your LED by hooking it up to a commercial battery. A commercial battery will be too powerful and will wreck the LED.
- Use the voltmeter or multimeter to check the voltage between the two electrodes. It will probably be less than 1 volt! That’s not enough to light the LED, which needs about 2 volts. Join together in groups of three or four lemons. Connect the lemons together in series (connect copper to zinc together with wire) and attach the ends to ONE bulb. Use the voltmeter to check the voltage between the free wires at the ends of the series.
Teacher Tip: The voltage will be extremely weak. You may need at least 3 lemons per battery for any visible movement to occur on the voltometer.
- Experiment with other fruits (e.g. oranges, grapefruits, apples, peaches, pears). Which ones produce the highest voltage? Why?
- Experiment with replacing the electrodes with two copper strips or two zinc strips and try to light the bulb. Measure the voltage and explain the results.
- Experiment with replacing the electrodes with different metals (e.g. iron and magnesium). Which combinations of electrodes give the highest voltage?
About the sticker
Artist: Jeff Kulak
Jeff is a senior graphic designer at Science World. His illustration work has been published in the Walrus, The National Post, Reader’s Digest and Chickadee Magazine. He loves to make music, ride bikes, and spend time in the forest.
Comet Crisp
T-Rex and Baby
Artist: Michelle Yong
Michelle is a designer with a focus on creating joyful digital experiences! She enjoys exploring the potential forms that an idea can express itself in and helping then take shape.
Buddy the T-Rex
Science Buddies
Artist: Ty Dale
From Canada, Ty was born in Vancouver, British Columbia in 1993. From his chaotic workspace he draws in several different illustrative styles with thick outlines, bold colours and quirky-child like drawings. Ty distils the world around him into its basic geometry, prompting us to look at the mundane in a different way.
Western Dinosaur
Time-Travel T-Rex
Related Resources
Electrical energy, electric motors are everywhere in your house, almost every mechanical movement that you see around you is caused by…, static electricity, have you ever rubbed a balloon on your head if you have, you may wonder why your hair…, related school offerings.

Electricity
We believe that now, more than ever, the world needs people who care about science. help us fund the future and next generation of problem solvers, wonder seekers, world changers and nerds..

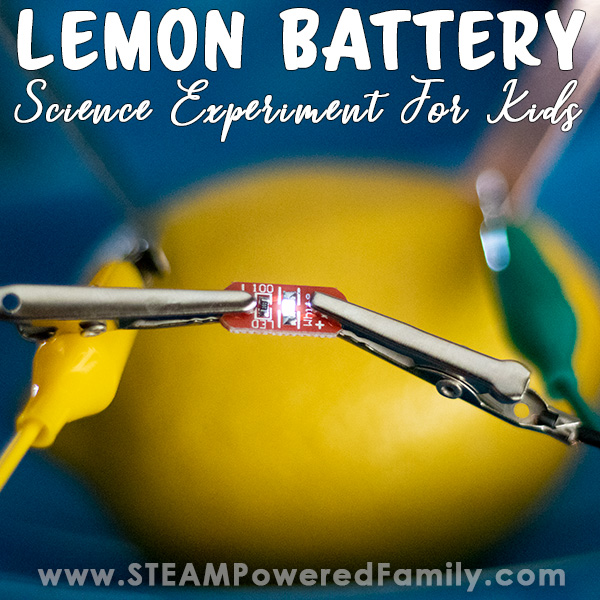
Lemon Battery Science Experiment
We love building circuits around here. From our very first Circuit Bugs creation to Potato Batteries , we have had a lot of fun over the years experimenting with low voltage experiments and electricity in our elementary science lessons. With summer here, that means lemons and lemonade. It also means it was time for us to create the favourite lemon battery science experiment.
How to Build a Lemon Battery
What you will discover in this article!
Disclaimer: This article may contain commission or affiliate links. As an Amazon Influencer I earn from qualifying purchases. Not seeing our videos? Turn off any adblockers to ensure our video feed can be seen. Or visit our YouTube channel to see if the video has been uploaded there. We are slowly uploading our archives. Thanks!
We often talk around here about the energy in nature and in everything around us. When we can power a light bulb with that energy it suddenly makes it very real for my kids. That energy isn’t just some crazy weird thing that I babble on about, it is this very real power that is showing itself right in front of them.
Normally our circuits are powered by batteries, but one day I convinced the kids we could power a light bulb with nothing but a potato. You should have seen the looks on their faces! Serious side eye was thrown my way.
Then, once they stopped straining their eyeballs, we built a potato battery and it worked! These kinds of science experiments for kids really stick with them. Why? Because it makes things real that they can’t otherwise see. Like the energy in our food.
Plus, when a child starts a science experiment with serious doubts, yet still achieves success, it powers up their curiosity!
So when we went grocery shopping and there was a huge pile of fresh, juicy looking lemons on display the kids asked to buy some for lemonade, but I knew we had another science experiment in our future.
Note: These food based battery experiments produce low voltage and are safe for older, responsible children to do under adult supervision.
How to Build a Lemon Battery Video
Watch as I go through the whole experiment step by step in our video tutorial. If you can’t see the video, please turn off your adblockers as they also block our video feed. Alternatively, you can also find this video on the STEAM Powered Family YouTube Channel .
Lemon Battery Materials
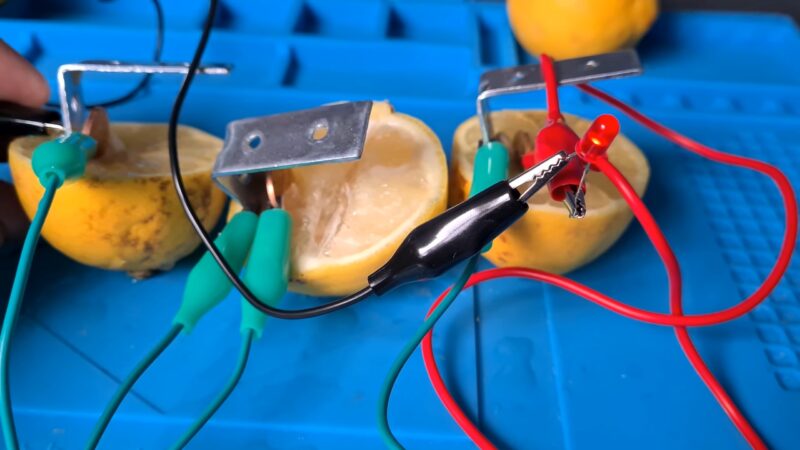
Lemons! You need at least 4 to create enough energy, but why not grab extras and experiment? Copper anode strip plates Zinc anode strip plates Alligator clips with wires (2 per cell, so minimum 8 if you are creating a 4 cell battery) LED light diodes Multimeter Knife and cutting board
Copper and Zinc plates are invaluable in our science experiments, but if you don’t have them, you can use copper pennies (the older the better) and zinc plated (aka galvanized) nails. Copper wire can also be used, and a search of your local hardware store is likely to produce other copper and zinc items you could test in your experiment.
The first step is to roll the lemons. Just like you would if you were about to eat or juice them. This releases the juices inside and we want our lemons as juicy as possible.
Start with one lemon and make a small cut through the peel on either end. It is very important that you place these far enough apart that the electrodes don’t touch.
Insert a copper plate on one side and a zinc plate on the other side.
Now using your multimeter test your energy levels.
We have energy!

Now it is time to start adding more cells (lemons) to our battery.
Repeat the above steps on a second lemon. Once you are finished use an alligator clip to connect the zinc plate on the first lemon to the copper plate on the second lemon.
Test your energy level with 2 cells (you will test by touching the copper plate on the first lemon and zinc on the second). Remember you are completing the circuit.
Now repeat the steps to add a third and fourth cell.
At 4 cells we are now registering more energy than 2 AA batteries, which we tested in our Potato Cell experiment .

Now it is time to hook up our light bulb!
Voila! Light!
The goal of making a lemon battery is to turn chemical energy into electrical energy, creating enough electricity to power a small LED light. You can also use limes, oranges, potatoes , pumpkins/squash , or other acidic foods.
How A Lemon Battery Works
How does a lemon battery work? The science behind how food can power a light bulb is really fascinating. Food has energy. With a lemon battery we are capturing that energy and using it to light up a LED. To do this we need electrodes to capture the energy from our electrolyte.
The zinc and copper plates are called electrodes, and the lemon juice is our electrolyte.
All batteries have a “+” (known as the cAll batteries have a “+” (known as the cathode) and a “-” (known as the anode) terminal. In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative anode. The zinc metal (our negative anode) reacts with the acidic lemon juice (mostly from citric acid) to produce zinc ions (Zn2+) and electrons (2 e-).
Electric current is created by the flow of atomic particles called electrons. Conductors are materials that allow electrons (and the electrical current) to flow through them. Electrons flow from the negative to the positive terminal.
So in our experiment electrons are flowing from our zinc plate, through the lemon juice to the copper plate. From there it goes into our alligator clip, along the wire, into the zinc plate on the next lemon, where it picks up more energy as it travels through that cell. It continues on, building energy with each additional cell we add. Until finally we have enough voltage to power a light bulb.
Volts (or voltage) is a measurement of the force moving the electrons through our lemon battery. The higher the voltage the more power the battery has, but higher voltage also means greater danger. Always remember to be careful and safe around electricity. Thankfully our lemon battery is very low voltage.
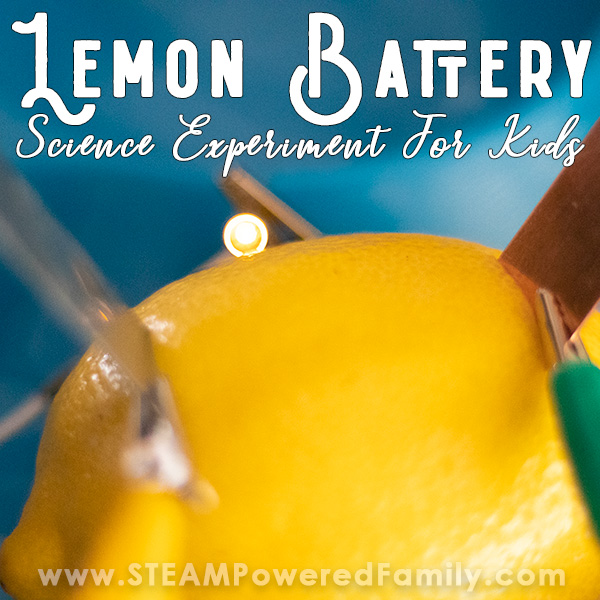
Troubleshooting
There are a number of things that can cause issues with your Lemon Battery.
First, make sure none of your electrodes are touching anything other that lemon and alligator clips. Also, ensure your alligator clips are placed near the peel of the lemon.
Did you roll your lemons? You want them juicy for this experiment to work.
Did you mix up any of your connections? Remember you always want to link “+” to “-“. On an standard LED light bulb the longer pin is the positive connection.
Does your LED bulb work? Test it on a coin battery to ensure your bulb works. It may be you have a faulty bulb.
Another area that can cause problems is the quality of your copper and zinc. You want your copper and zinc to be as pure as possible so it can conduct the electrons without any interference. This is one of the reasons I suggest investing in proper plates, so you know the quality of your materials when conducting experiments.
Finally, these food based batteries dimly light up the LED. If you hook your LED up to a regular battery, it will glow much brighter.
More Fruit Battery Experiments
So now we have made both a lemon battery and a potato battery, which one is better? Both were able to light up our LED light bulbs, so in that sense they are both successful. However, the potato battery was definitely a lot more work. So if you are looking for a quicker experiment, the lemon battery is faster and easier. However, both have significant opportunities for learning and would make great science fair projects. Why not do both yourself and see what you think?
And in the fall, don’t forget to make a battery with pumpkins and squash ! The concept is similar to Lemon Batteries but with a Autumn/Halloween theme.
Want to dig in more? Try this experiment with other citrus fruit such as oranges or lime or grapefruit. You can also combine a variety of fruits to see which combination makes the best fruit battery.
How to Reuse Lemon Battery Cells
This lemon science project is a ton of fun but once you are done, what can you do with the lemons? It seems like such a waste to throw them out. We have two really cool projects to do next with your lemons!
Check out the gorgeous lemon volcano we created here after building our lemon batterie s!

Another great project with these lemons is to make Lemon Oobleck for a fun, summery sensory project.

More Electricity Experiments

5 Days of Smart STEM Ideas for Kids
Get started in STEM with easy, engaging activities.

By Audience
- Therapist Toolbox
- Teacher Toolbox
- Parent Toolbox
- Explore All
By Category
- Organization
- Impulse Control
- When Executive Function Skills Impair Handwriting
- Executive Functioning in School
- Executive Functioning Skills- Teach Planning and Prioritization
Adults With Executive Function Disorder
- How to Teach Foresight
- Bilateral Coordination
- Hand Strengthening Activities
- What is Finger Isolation?
- Occupational Therapy at Home
- Fine Motor Skills Needed at School
- What are Fine Motor Skills
- Fine Motor Activities to Improve Open Thumb Web Space
- Indoor Toddler Activities
- Outdoor Play
- Self-Dressing
- Best Shoe Tying Tips
- Potty Training
- Cooking With Kids
- Scissor Skills
- Line Awareness
- Spatial Awareness
- Size Awareness
- Pencil Control
- Pencil Grasp
- Letter Formation
- Proprioception
- How to Create a Sensory Diet
- Visual Perception
- Eye-Hand Coordination
- How Vision Problems Affect Learning
- Vision Activities for Kids
- What is Visual Attention?
- Activities to Improve Smooth Visual Pursuits
- What is Visual Scanning
- Classroom Accommodations for Visual Impairments

Sensory Processing Disorder Checklist
- Free Resources
- Members Club
- Executive Functioning Skills , Eye Hand Coordination , Fine Motor Skills , Functional Skills , Occupational Therapy
Lemon Battery Science Fair Project
Colleen beck otr/l.
- by Colleen Beck OTR/L
- February 2, 2023
Lemon STEM is such a fun way to explore concepts and this lemon battery science fair project is a winner! By using a lemon and a few other materials, you can discover the chemical process of moving electric current through a lemon to create a lemon battery. This lemon battery is a discovery activity that makes a great science fair project because the experiment is a powerful tool for discussing science and exploration in kids. Plus, this lemon science experiment is easy to do (and clean up)!
Lemon Battery Science Fair Project
One of the best benefits to making this lemon battery science fair project is that it’s an easy way to learn about electrodes, electrons, and the chemical reaction required to fire up a battery. As an occupational therapist and mom, I love the other skill-building opportunities with this project too:
- Fine motor skills
- Engineering and building
- Planning, prioritization, task completion and other executive functioning skills
- Using different materials found around the home (accessibility!)
- STEM activity
When kids build the lemon battery, they are building so many skills!
Introduce them to creativity through STEM? Sounds great! Encourage my children to get excited about science and math? YES! Unleash natural potential in my girls by experiencing science projects? I like it.

And the best for me, was watching my girls do this together. The baby saw her big sister in safety goggles as she learned about cathodes and electrolytes…and has been wearing the goggles every day since. Seeing them inspire each other was just awesome.
We were making lemon powered batteries!
What is a lemon battery?
A lemon battery is a simple science experiment kids can do to explore concepts of conduction and reaction. In the lemon-powered battery experiment, kids can see how electrolytes are conducted through the lemon and wires in order to power a light or clock.
The experiment is simple set up, easy, and a fun way to explore science!
You move
Food Battery Experiment
When I first showed the girls the items and explained what we were doing, they were very excited about lemon electricity! I was surprised to read that only 1 in 1,000 girls pursue STEM careers, especially considering that out us us three sisters, two of us are in the health/science field. Encouraging my girls to explore interests in science is important to me and I was super pumped to get my girls excited about our science experiment…and the enthusiasm was catchy!
We used a lemon in our fruit battery, but you could use any citrus fruit to make a citrus battery…oranges, grapefruit, lemons, and limes all work equally well in this kid-made battery experiment.
Other fruits and vegetables can be used in this experiment too. It might be fun to explore which food is the best conductor for passing electrical energy. Try these fruits and veggies:
Which food battery works fastest? Which is the strongest conductor? Which food battery will work the quickest? These are all fun science fair experiments to try!
Lemon Battery Science Experiment
We created the lemon-powered battery, but then used the battery in a STEM activity by adding engineering and math to the mix.
To make the lemon battery, you’ll need a few items”
- LED Bulb or a small clock, light bulb, etc.
- 4 Lemons
- Knife to cut the lemon
- Alligator Clips on Lead wires
- Zinc Nails
- Copper Wire (or a penny)
- Alcohol wipes
- Recording sheet
You can also try different metals instead of the copper penny. Try a galvanized nail, Copper electrodes, Aluminum foil, metal strip, or other types of metal material.
Also note that you should have adult supervision for this activity because cutting the food with a knife can be tricky!
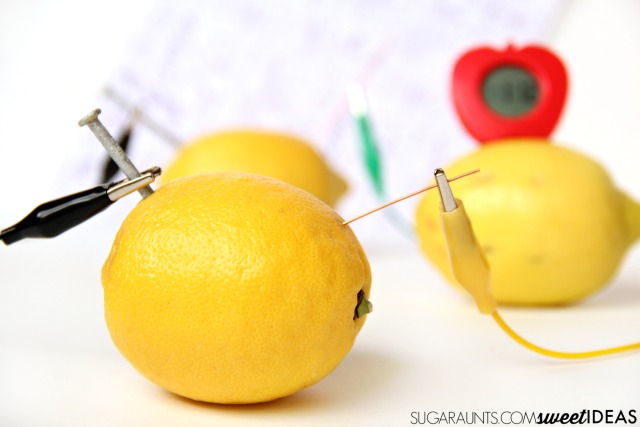
And we got started on our STEM project. The instructions are easy to follow images below.

How to make a lemon battery:
- Start with a clean surface.
- Use the knife to make a small cut in the lemon’s surface. This will be used to push the nail and copper penny into the lemon more easily.
- Use an alcohol wipe to clean off the lemon or other food item used for the battery.
- Press the nail and copper item (penny, copper wire, etc.) into the lemon. Make sure the metal goes all the way past the peel if using a citrus fruit. You can use more than one lemon too: Push a nail into one lemon and the copper into the other lemon.
- Attach the alligator clips to the nail and to the copper item. Connect the ends of one alligator clip wire to a galvanized nail in one lemon and then the other end of the alligator clip wire to a piece of copper in another lemon. When you are finished you should have one nail and one piece of copper unattached.
- Finally, connect the unattached piece of copper to the unattached nail to the positive and negative connections of your light. The lemon will act as the battery.

- Following the instructions, my eight year old build a lemon powered battery that lit up a light bulb. We tried a few more experiments, like the mini fruit clock that came in the kit. We used it to make a lemon clock with the circuits!

This Lemon STEM activity is a great fine motor STEM idea. By pushing the nails and pennies into the lemon, cutting with a knife, and clipping alligator clips, you are building fine motor strength in a functional task.
Add a few other ways to support lemon stem too: Use wooden skewers to build an elevated lemon battery.
Provide a handful of wooden skewers and ask the children to build a contraption that is strong enough to hold a lemon up off the ground. This can take a bit of creativity and trial and error, so be sure use plenty of paper towels or a wash cloth to wipe off lemon juice.
- We pulled out some bamboo skewers and created a sky high lemon battery and lit up the light bulbs using engineering in our STEM activity.
- Try building a clock tower with the skewers and a lemon. Explore how to make electricity run the clock even when it’s elevated or in different weather conditions like rain or freezing temperatures.
- With all of the zinc nail-punctured holes in our lemons, we HAD to squeeze the juice. We tried to see if we could create a lemon clock using just the lemon juice in a cup. It worked!
- After the lemons were juiced, we tried to make another light bulb glow using the rinds. This time the lights did not brighten and we decided it was because the electrolytes were squeezed away into our lemon juice and the current stopped at the rind.
Next, we used wooden skewers to create a clock tower. Press the skewers into the lemons and create a tower. You’ll need to figure out how to get the clock tower to stand without toppling, and using lemons as the base or at the connecting points. These lemons can also be connected to one another with the alligator clips, wires, and pennies or nails to conduct through the whole tower.
After all of these experiments, we were feeling a little thirsty. Non-lemon powered light bulbs went off and so my four year old had a bright idea to make lemonade. We added water and sugar and drank away the electrolytes!
It was so much fun to see my girls working together, encouraging each other, (not fighting), and being inspired in science. Someday they might look back at our experiment day and laugh at drinking their science experiment, but I’ll remember the sticky crumbs on the table, the goggles on the one year old, and the fun we all had learning together.

Colleen Beck, OTR/L has been an occupational therapist since 2000, working in school-based, hand therapy, outpatient peds, EI, and SNF. Colleen created The OT Toolbox to inspire therapists, teachers, and parents with easy and fun tools to help children thrive. Read her story about going from an OT making $3/hour (after paying for kids’ childcare) to a full-time OT resource creator for millions of readers. Want to collaborate? Send an email to [email protected].

More Posts Like This

- Occupational Therapy , Sensory

- Development
Retained Primitive Reflexes & Child Development

- Development , Eye Hand Coordination , Occupational Therapy Activities
Crossing Midline Activities

- Attention , Executive Functioning Skills , Occupational Therapy
Quick Links
Sign up for the ot toolbox newsletter.
Get the latest tools and resources sent right to your inbox!
Get Connected

- Want to read the website AD-FREE?
- Want to access all of our downloads in one place?
- Want done for you therapy tools and materials
Join The OT Toolbox Member’s Club!
Get Your ALL ACCESS Shop Pass here →

Lemon Battery Experiment
What can you power with a lemon battery ? Grab some lemons and a few other supplies, and find out how you can turn lemons into lemon electricity! Even better, make this into a lemon battery science project with a few simple ideas. We love hands-on and easy-to-set-up science experiments for kids .
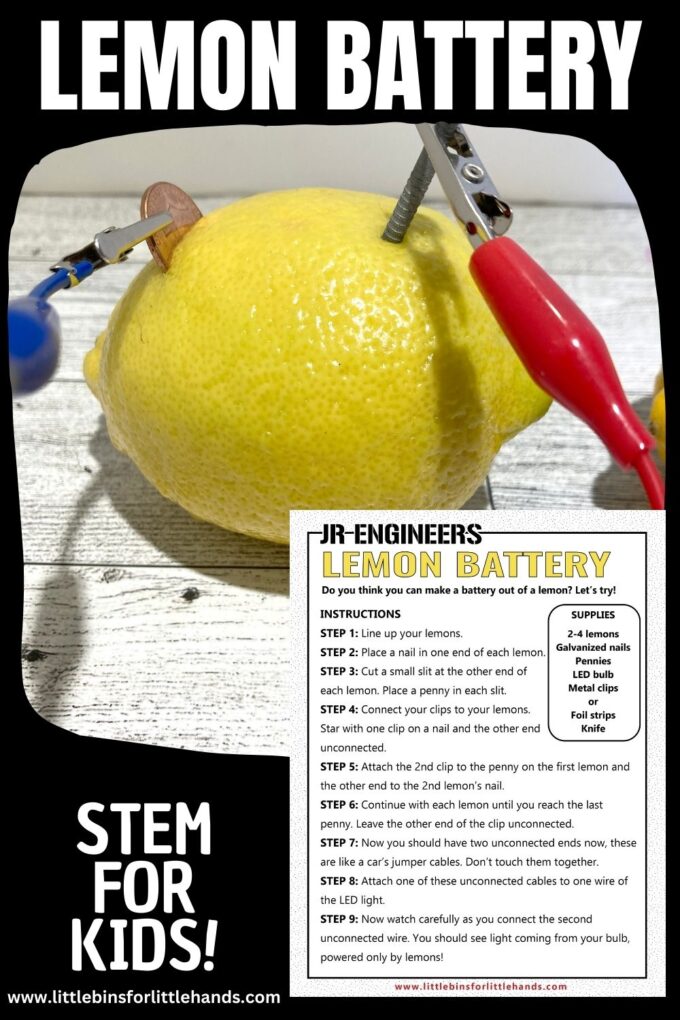
Lemon Battery Experiment Set Up
Remember to supervise kids during this experiment, especially when handling the nails and using the wires. Enjoy exploring the world of science and electricity!
- 2 to 4 lemons
- Galvanized nails
- Metal clips (Amazon Affiliate link) or Foil strips
Have leftover lemons? Try this apple oxidation experiment , a lemon volcano , invisible ink , or even make fizzy lemonade !
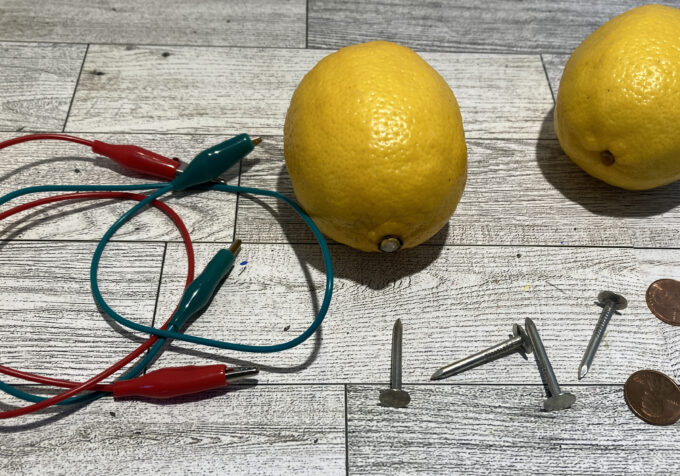
Instructions:
STEP 1: Line up your lemons.
STEP 2: Place a nail in one end of each lemon.
STEP 3: Cut a small slit at the other end of each lemon. Place a penny in each slit.

STEP 4: Connect your clips to your lemons. Start with one clip on a nail and the other end unconnected.
STEP 5: Attach the 2nd clip to the penny on the first lemon and the other end to the 2nd lemon’s nail.
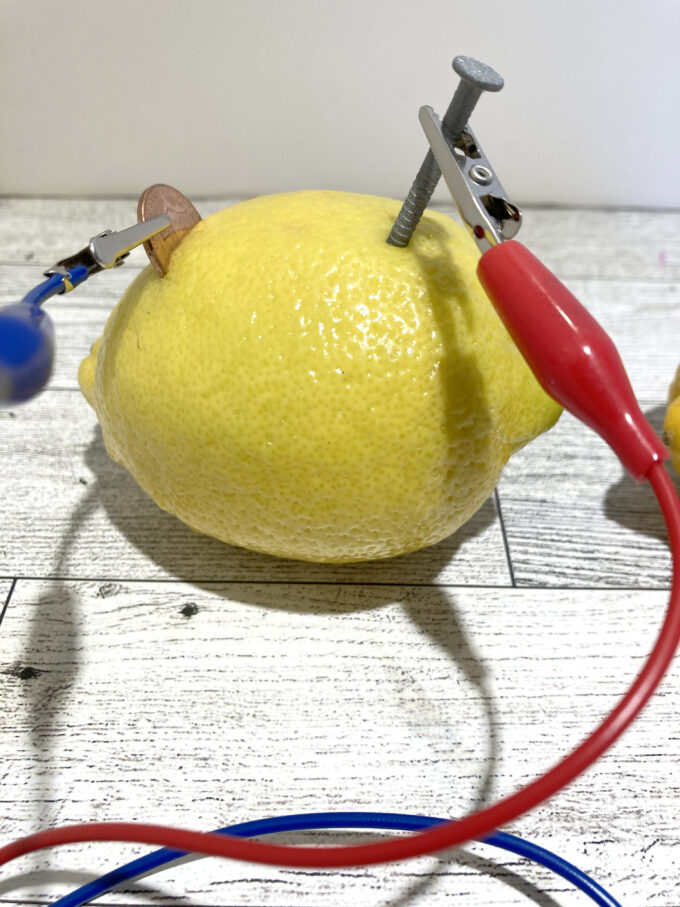
STEP 6: Continue with each lemon until you reach the last penny. Leave the other end of the clip unconnected.
STEP 7: Now you should have two unconnected ends; these are like a car’s jumper cables. Don’t touch them together!
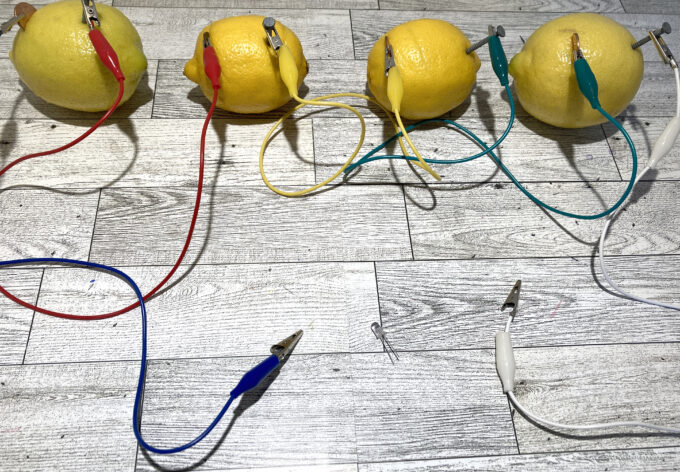
STEP 8: Attach one of these unconnected cables to one wire of the LED light.
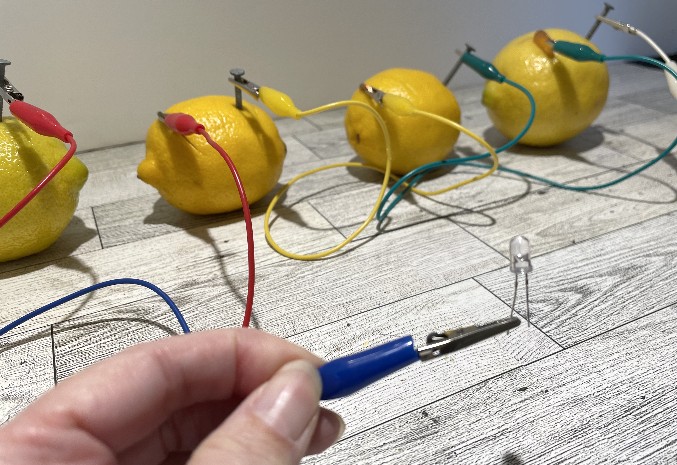
STEP 9: Now watch carefully as you connect the second unconnected wire. You should see the light coming from your bulb, powered only by lemons!
Can you do this with other fruit and vegetables? See our potato battery experiment .

Free Printable Lemon Battery Project
How Does a Lemon Battery Produce Electricity?
A lemon battery is a type of battery that you can make at home using a lemon and some simple materials. It works through a process called electrolysis. The lemon juice acts as an electrolyte, which is a liquid that can conduct electricity.
Also check out how we powered a digital clock with a pumpkin battery!
When the penny and the nail are inserted into the lemon, they become the positive and negative terminals of the battery. The penny is made of copper and acts as the positive electrode, while the nail is made of zinc and acts as the negative electrode.
The zinc and copper electrodes are immersed in the electrolyte lemon juice, and the electrons from zinc atoms flow to the copper atoms which causes a small electrical current. This current is then able to power a small device, such as a light bulb.
Lemon batteries are not a practical source of power to use all the time but they are a simple and fun way to learn about how electricity works.
Discover more easy physics experiments for kids!
Make a Lemon Battery Science Fair Project
Want to turn this lemon battery into a cool lemon battery science project? Check out these helpful resources below to set up a fantastic science demonstration.
- Easy Science Fair Projects
- Science Project Tips From A Teacher
- Science Fair Board Ideas
- Variables In Science
How to Apply the Scientific Method
Apply the scientific method to this lemon battery project and turn it into a lemon battery experiment by choosing a question to investigate.
For example, does increasing the number of lemons increase the amount of electricity produced? Or which one powers a light bulb for longer, potato battery or lemon battery?
If you want to set up an experiment with several trials, pick one thing to change, such as the number of lemons! Don’t change everything! You need to change the independent variable and measure the dependent variable .
You can also get kids started by writing down their hypotheses before diving into the experiment. What do they think will happen when you increase the number of lemons used?
After performing the experiment, kids can conclude what happened and how it matched their initial hypotheses. You can always change a hypothesis upon testing your theory!
More Fun Science Experiments to Try
- Magic Milk Experiment
- Egg In Vinegar Experiment
- Skittles Experiment
- Freezing Water Experiment
- Growing Borax Crystals
- What Dissolves?

Printable STEM Pack for Kids
80+ Doable Engineering Projects in one convenient pack!
- Full instructions with sample images
- Activity-specific instruction sheets
- Data Collection Sheets
- Questions for Reflection
- Architecture Building Cards: Try the tallest tower challenge
- Bridge Building Cards: Explore different types of bridges to build your own.
- Paper Chain STEM Challenge: Who can make the longest chain? Great icebreaker or quick challenge!
- 3 Little Pigs Architectural Pack: Design a house that won’t blow away!
- Great marshmallow challenge: A classic challenge kids love!
- Real-world STEM challenge lesson but don’t know where to start? Our easy-to-follow template shows the steps!
- What’s the difference between a scientist and an engineer?
- Crossword and word search with engineering vocabulary.
- Engineering vocabulary cards
- Design a one-of-a-kind invention and write about it with this 5-page activity!

Subscribe to receive a free 5-Day STEM Challenge Guide
~ projects to try now ~.

- Skip to main content
- Keyboard shortcuts for audio player

- LISTEN & FOLLOW
- Apple Podcasts
- Amazon Music
- Amazon Alexa
Your support helps make our show possible and unlocks access to our sponsor-free feed.
When Life Gives You Lemons...Make A Battery

Emily Kwong

Rebecca Ramirez
Madeline K. Sofia

Electrical circuit with lemons. A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb. Science Photo Libra/Getty Images hide caption
Electrical circuit with lemons. A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb.
We're going "Back To School" today, revisiting a classic at-home experiment that turns lemons into batteries — powerful enough to turn on a clock or a small lightbulb. But how does the science driving the "lemon battery" show up in those household batteries we use daily?
Short Wave host Maddie Sofia and reporter Emily Kwong speak with environmental engineer Jenelle Fortunato about the fundamentals of electric currents and the inner workings of batteries.
Fortunato is a postdoctoral researcher at North Carolina State University studying materials for electrodes that can be used in solid-state batteries.
A few years ago, she brought the "lemon battery" to classrooms through Penn State's Science U program. Middle schoolers got particularly invested in the experimental possibilities.
"They hooked up like 20 lemons, three cups of lemon juice, an apple, three different light bulbs and a buzzer buzzing. And it was... it was chaos...I was in awe. Leave it to kids to come up with something like that," Fortunato said.
You can build your very own lemon battery using Science U's design here , written by Fortunato and Christopher Gorski of Penn State College of Engineering.
A reminder: Do NOT play with household batteries. Be safe out there, scientists!
You can read more about Fortunato's research here .
This episode was produced by Rebecca Ramirez, edited by Viet Le and fact-checked by Rasha Aridi. J. Czys and Josh Newell were the audio engineers. Special thanks to Short Wave listener Violet Thomas for inviting us to dig deeper into battery science.
Sciencing_Icons_Science SCIENCE
Sciencing_icons_biology biology, sciencing_icons_cells cells, sciencing_icons_molecular molecular, sciencing_icons_microorganisms microorganisms, sciencing_icons_genetics genetics, sciencing_icons_human body human body, sciencing_icons_ecology ecology, sciencing_icons_chemistry chemistry, sciencing_icons_atomic & molecular structure atomic & molecular structure, sciencing_icons_bonds bonds, sciencing_icons_reactions reactions, sciencing_icons_stoichiometry stoichiometry, sciencing_icons_solutions solutions, sciencing_icons_acids & bases acids & bases, sciencing_icons_thermodynamics thermodynamics, sciencing_icons_organic chemistry organic chemistry, sciencing_icons_physics physics, sciencing_icons_fundamentals-physics fundamentals, sciencing_icons_electronics electronics, sciencing_icons_waves waves, sciencing_icons_energy energy, sciencing_icons_fluid fluid, sciencing_icons_astronomy astronomy, sciencing_icons_geology geology, sciencing_icons_fundamentals-geology fundamentals, sciencing_icons_minerals & rocks minerals & rocks, sciencing_icons_earth scructure earth structure, sciencing_icons_fossils fossils, sciencing_icons_natural disasters natural disasters, sciencing_icons_nature nature, sciencing_icons_ecosystems ecosystems, sciencing_icons_environment environment, sciencing_icons_insects insects, sciencing_icons_plants & mushrooms plants & mushrooms, sciencing_icons_animals animals, sciencing_icons_math math, sciencing_icons_arithmetic arithmetic, sciencing_icons_addition & subtraction addition & subtraction, sciencing_icons_multiplication & division multiplication & division, sciencing_icons_decimals decimals, sciencing_icons_fractions fractions, sciencing_icons_conversions conversions, sciencing_icons_algebra algebra, sciencing_icons_working with units working with units, sciencing_icons_equations & expressions equations & expressions, sciencing_icons_ratios & proportions ratios & proportions, sciencing_icons_inequalities inequalities, sciencing_icons_exponents & logarithms exponents & logarithms, sciencing_icons_factorization factorization, sciencing_icons_functions functions, sciencing_icons_linear equations linear equations, sciencing_icons_graphs graphs, sciencing_icons_quadratics quadratics, sciencing_icons_polynomials polynomials, sciencing_icons_geometry geometry, sciencing_icons_fundamentals-geometry fundamentals, sciencing_icons_cartesian cartesian, sciencing_icons_circles circles, sciencing_icons_solids solids, sciencing_icons_trigonometry trigonometry, sciencing_icons_probability-statistics probability & statistics, sciencing_icons_mean-median-mode mean/median/mode, sciencing_icons_independent-dependent variables independent/dependent variables, sciencing_icons_deviation deviation, sciencing_icons_correlation correlation, sciencing_icons_sampling sampling, sciencing_icons_distributions distributions, sciencing_icons_probability probability, sciencing_icons_calculus calculus, sciencing_icons_differentiation-integration differentiation/integration, sciencing_icons_application application, sciencing_icons_projects projects, sciencing_icons_news news.
- Share Tweet Email Print
- Home ⋅
- Science ⋅
- Physics ⋅
- Electricity and Magnetism: What Are They & Why Are They Important?
How to Build a Simple Lemon Battery

Easy 10 Minute Science Projects
Lemons make us pucker, but the same property in lemon juice that creates a sour flavor--acid--is what gives lemons battery power. The acid in lemons works like regular battery acid to create an electrolyte reaction with the metals that produces power. Simply make a positive and negative electrodes that connect to the lemon acid with a couple household items, and test. If you have a multimeter handy, you can visibly measure the lemon power output in this simple voltaic battery.
Roll the lemon on a table gently to release the juices inside without breaking the skin.
Push the copper wire in the lemon through the skin about ½ inch. If you don’t have copper wire, make a slit in the skin using a knife and insert a copper penny instead.
Insert the nail or a steel paper clip into the lemon as close to the copper wire or penny as possible without touching.
Place your tongue on the penny and the nail or paperclip next to it, or on the tips of the copper wire and the nail/paperclip at the same time.
You should feel a slight tingling on your tongue that means the lemon battery is working.
Measure voltage if you have a multimeter or volt meter available. Attach the meter's positive and negative wire end clips to the copper wire or penny, and the galvanized nail or paperclip. Then check the voltage readout to see if the lemon battery has power.
Things You'll Need
- If the lemon battery does not work, try switching the penny for a pure copper wire that will conduct the energy better.
- Add more lemon batteries connecting between electrodes with wires to increase the voltage enough to run an LED light, digital watch or basic calculator.
- Building a lemon battery is a good science experiment for home-schooled kids grades 3-8. Print off a lab sheet or experiment guide online such as one from the National Engineers Week Foundation.
- Do not do this experiment with children under 5 because they could choke on small parts such as the penny or paperclip.
Related Articles
How to make a potato lamp, how to make a simple dry cell battery, how to make electricity for a science fair project..., how to build a clorox bleach battery, how to make a light bulb work with a battery, how to make a battery with coke & vinegar, how to light a flashlight bulb using potatoes, science project on electricity in a potato, how to make electricity using an orange, how to make a negative charge magnet, what fruits & vegetables conduct electricity, how to make a powerful dc electromagnet, how does a potato clock work, how to extract lemon oil, why do citrus fruits produce electricity, what are some possible materials you could use to make..., how to build an emf detector, how to use vinegar & salt to make a penny disappear, potato light bulb experiment for kids.
- PBS Kids: Lemon Battery
About the Author
Roxanne McHenry has written online marketing articles and courses for Web publications including Affiliate Classroom and Web Pro News since 2002. McHenry has a B.A. in Japanese language and literature, and lived and worked in Japan as a teacher and technical translator.
Photo Credits
Hemera Technologies/AbleStock.com/Getty Images
Find Your Next Great Science Fair Project! GO
- Experiments
Lemon battery
Light up a diode... with a lemon!

Put on protective gloves and eyewear.
Conduct the experiment on the tray.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
The diode doesn’t light up. what to do.
First, ensure that the plates in a lemon do not touch each other.
Second, check the connection between the crocodile clips and the metal plates.
Third, make sure that the diode is connected correctly: the black crocodile clip should be connected to a short “leg”, and the red clip to a long one.
Importantly, crocodile clips shouldn’t touch the other “leg” to avoid short-circuiting!
Juice is hissing around the magnesium plate. Is it normal?
Everything is alright. Magnesium is an active metal, and it reacts with citric acid. The reactions yields magnesium citrate and releases hydrogen.
Step-by-step instructions
- Take 2 magnesium plates from the vial marked “Mg.”
- Take 2 crocodile clips: 1 black and 1 white. Clip the crocodile clips—black and white—onto the magnesium plates.
- Take 2 copper plates from the vial marked “Cu.”
- Connect one copper plate to a free end of the white crocodile clip wire. Connect the other copper plate to a free end of the red crocodile clip wire.
- Slice a lemon in half. Insert the copper and magnesium plates into one lemon half at a distance of about 1 cm apart from each other. Repeat with the other two plates using the other lemon half. Make sure that the plates do not touch each other.
- Take a light emitting diode. Connect a free end of the red crocodile clip wire to a long leg of the diode. Connect a free end of the black crocodile clip wire to a short leg of the diode. The diode will light up!
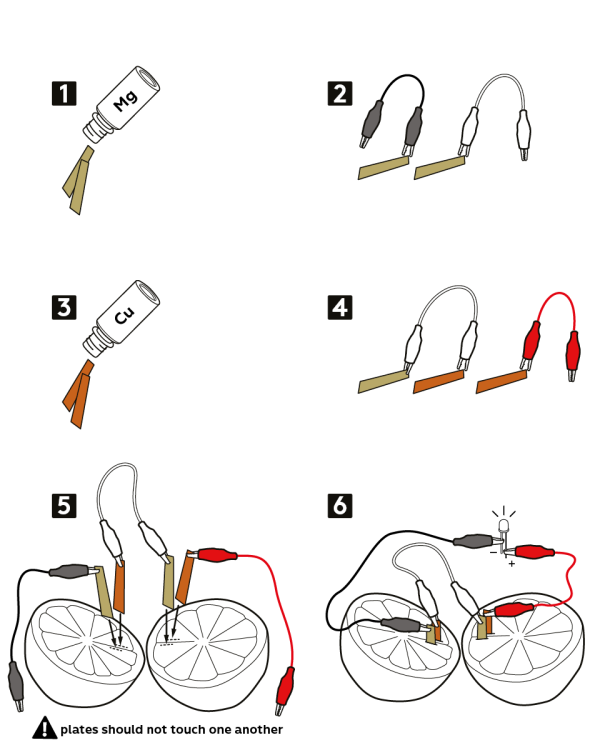
Dispose of solid waste together with household garbage. Pour solutions down the sink. Wash with excess of water.
That’s interesting!
What is diode, and how does it work.
Diode is a tiny device that can conduct electricity (in one direction) and sometimes turn this energy into useful work. In our case, we will deal with a light-emitting diode (LED): it glows when passing through electrical current.
The base of all modern diodes is a semiconductor. The latter is a special material with quite poor electroconductivity that can, however, be increased – for instance, by heating. By the way, what is electroconductivity? It is ability of a material to conduct electrical current.
Interestingly, unlike a regular conductor, any diode contains two “types” of semiconductors. Even the word diode (from the Greek δίς) indicates that it comprises two elements: an anode and a cathode.
An anode in a diode is made of a semiconductor that contains so-called holes. They are the void regions that can be filled with electrons. These holes may be imagined as empty shelves designed specifically for electrons. Moreover, these “shelves” may to a certain degree freely move throughout the anode. A cathode in a diode is also made of a semiconductor. However, this second semiconductor is different: it contain excess of electrons that, again, can almost freely move throughout the cathode.
Notably, this construction of a diode allows electrons to freely pass through a diode in one direction, but prohibits their movement in the opposite direction. When electrons move from a cathode toward an anode, then on the border between these parts “free” electrons in the cathode meet with electron vacancies (“shelves”) in the anode. There, electrons gladly occupy these vacancies, allowing the current to move further. You can see this process in the video below.
Now, let’s imagine that electrons have to move in the opposite direction. They have to leave their cozy shelves and move into the material that has no shelves at all! Obviously, this way is no gain for electrons, and thus the current doesn’t flow in that direction.
Therefore, any diode may work as some sort of a check valve for electricity: it passes through a diode in one direction and cannot pass through in the opposite direction. This unique property provided for the use of diodes in electronics. Any computer, smartphone, laptop of a tablet has a processor containing millions of microscopic diode-like devices called transistors.
Light-emitting diodes, in turn, are employed in lighting and indicating. To make a diode that produces light, they thoroughly select its semiconductor components. In a certain combination of selected semiconductors, transfer of electrons from a cathode to vacancies in an anode is accompanied by emission of a photon, i.e. a portion of light. For various semiconductors, glow color is different. An important feature of diodes, in comparison with other electrical sources of light, is their safety and high efficiency, or degree of converting electrical current into light.

Dozens of experiments you can do at home
One of the most exciting and ambitious home-chemistry educational projects The Royal Society of Chemistry

How to Make a Lemon Volcano Science Experiment
This post may contain affiliate links.
A lemon volcano is a fantastic hands-on science experiment that brings a little zest to learning! This activity combines a simple chemical reaction with sensory play! It will excite and engage young learners while introducing them to some basic scientific principles. I love that you can bring science to life with everyday materials like lemons!

Whether you’re homeschooling, teaching in the classroom, or simply looking for a fun educational activity at home, lemon volcanoes are popular with kids of all ages. In this post, I am going to dive into the science behind it, give you some step-by-step instructions, and tell you the educational benefits of this extra fun science experiment.
The Science Behind Lemon Volcanoes
The lemon volcano is an acid-base reaction. Lemons contain citric acid, a naturally occurring weak acid. When you mix this with a base, like baking soda (sodium bicarbonate), a chemical reaction occurs. This reaction produces carbon dioxide gas, which creates the fun fizzing and bubbling effect that looks like a mini volcano erupting.

As the lemony citric acid reacts with the baking soda, carbon dioxide bubbles form and create a foamy eruption. Food coloring can be added to enhance the visual effect, making the reaction even more exciting. Add a little dish soap for an even foamier reaction!

How to Make a Lemon Volcano

Step-by-Step Instructions:

- Add Food Coloring (Optional): For a colorful volcano, drop a few drops of food coloring inside the lemon. Red, orange, or yellow work well to mimic a real volcanic eruption! We used blue because I loved the cool contrast! Use all of the colors in different sections and make a rainbow of volcanoes!
- Add Dish Soap (Also optional): To make the reaction frothier, squeeze a little dribble of dish soap on top of the lemon pulp.

Volcano Variations:
Try the experiment with a different type of citrus fruit such as limes, or grapefruit. Make an apple volcano or pumpkin volcano using baking soda and vinegar.
Educational Benefits of the Lemon Volcano Experiment

Intro to Chemical Reactions: Lemon volcanoes are a fun and easy way to introduce kids to chemical reactions. They can observe how acids (citric acid) and bases (baking soda) react to form new substances, such as carbon dioxide. This can give a foundation for understanding more complex chemistry later on.
Sensory and Hands-on Learning: We love all kinds of hands-on learning here! This activity engages many senses—sight, smell, touch, and even hearing (bubbling sounds). Involving many senses increase the learning.
Critical Thinking: You can encourage children to come up with a hypothesis and make predictions about what will happen when baking soda is added to the lemon. Will it fizz right away? Will more soap create bigger bubbles? Asking questions like this get kids thinking and helps them develop a scientist mindset! Turn it into a science fair project.
Fine Motor Skills: Working with the lemons, squeezing juice, and stirring the mixture helps young children develop fine motor skills and hand-eye coordination.
Encourages Curiosity and Exploration: Once children see their first fizzy eruption, they’re going to want to try it again. This curiosity can lead to more questions and experimentation (like with other citrus fruits!). It can really help kids develop a love for science and exploration.
Simple STEM Learning : This activity is a great introduction to basic STEM principles. It allows children to explore cause and effect, make observations, and apply basic math concepts (like measuring ingredients).
I hope you’ll give these lemon volcanoes a try! They combine science and fun, making them the perfect activity to spark curiosity and excitement in children. Whether you’re looking to enrich your homeschool curriculum or just want a fun activity for a rainy afternoon, lemon volcanoes will definitely bring some amazing smelling excitement to your day!
See More Sensory Rich Science Experiments:
Erupting Volcano Slime
Lemon Battery Experiment
How to Make an Awesome Volcano Science Project
Tapioca Pearl Sensory Play
Five Senses Activities for Kids
Former school teacher turned homeschool mom of 4 kids. Loves creating awesome hands-on creative learning ideas to make learning engaging and memorable for all kids!
Similar Posts

Solar Powered LEGO Car

Story Starter Blocks for Creative Writing

5 Sound Wave Experiments for Kids
Is it conductive or not stem conductivity experiment.

100 Art Projects for Kids ~ Inspired by Famous Artists

The Internet and Education
Leave a reply cancel reply.
You must be logged in to post a comment.

IMAGES
VIDEO
COMMENTS
Use a lemon battery to power a small electrical device, like an LED. The lemon battery experiment is a classic science project that illustrates an electrical circuit, electrolytes, the electrochemical series of metals, and oxidation-reduction (redox) reactions.The battery produces enough electricity to power an LED or other small device, but not enough to cause harm, even if you touch both ...
If you are using zinc as an electrode, scratch its surface lightly to expose a fresh surface. Roll the lemon gently on a table to loosen the soft pulp and allow the juice to flow inside. Be careful not to rupture the skin of the lemon, though. Insert the copper wire by 1 inch into the lemon. After ensuring that your tongue is moist with saliva ...
Procedure. Place the lemon on its side on a plate and have an adult carefully use the knife to make a small cut near the middle of the lemon (away from either end). Make the cut about two ...
Create a lemon battery with the following steps: Begin by cutting the lemon in half and giving it a slight squeeze to release some juice. Insert the zinc nail into one lemon half, ensuring it avoids contact with the peel. In the other lemon half, insert the copper coin, making sure it remains peel-free.
Method & Materials. You will use a lemon, a penny or copper wire, a galvanized nail or zinc strip, and some alligator wires to create your lemon battery. After setting up the battery, you will connect the wires to a small light bulb to see if it lights up. You can even test the voltage with a voltmeter. You will need a lemon, paper towels, a ...
EXPERIMENT STEPS. Step 1: Cut 2 small slits in the skin of both the lemon and the potato. Make the slits are a few inches apart. Step 2: Push the copper and zinc strips into the slits in each piece of produce. Make sure the rods do not touch each other. Step 3: Connect an electrical wire to the end of each metal strip.
A red LED typically needs a voltage of 1.2-1.6 V, so we need more power to light the bulb. Follow steps 3-5 to make 3 or 4 more lemon cells. (Optional) If you have a multimeter, check each lemon battery to make sure it generates voltage and current. Connect the lemon batteries together using the wire test leads.
Hands-on Chemistry Activities Build a Lemon Battery, At-Home, Page 1 . Build a Lemon Battery At-Home . A lemon on its own is not a battery. But add electrodes, make a path for electrons to move, and you have all the basic elements of a battery. Build your own lemon battery and feel energized when you juice up a small LED with electricity!
To make a series of (6) connected lemon batteries cut (6) roughly 3"-4" long pieces of copper wire. Wrap each piece of wire around a galvanized nail. Leave 2" of the nail unwrapped. Place one copper wrapped galvanized nail in the first lemon/lime. Place a 2″ piece of copper wire in the other end of the lemon/lime.
Instructions. Put the lemon on a plate, on its side, and carefully use the knife to make a small cut near the middle of the lemon (away from either end). Make the cut about 2 cm long and 1 cm deep. Make a second, similar cut, parallel to the first, so the cuts are about 1 cm apart.
Procedure. Ask your grown-up to use the wire strippers to first strip about 2 1/2 inches of plastic insulation off the copper wire. Then, request that the grown-up clip that piece of stripped wire off of the main roll. Carefully straighten the steel paper clip. Use the wire clippers to cut it to the same length as your copper wire.
3. Make two slits on the top of the lemon, half an inch apart. 4. Insert a penny (or copper coin) into one slit and the zinc coated nail into the other slit. 5. Connect to a volt meter to see if voltage is produced by touching leads of voltmeter to both the penny and the zinc.
Using this activity, we cannot make a high voltage current. Let us learn how lemon fruit generates low voltage electricity. Step-1: As a first step, pick three to four fresh lemons and place them side by side intact. And give two vertical cuts about half-inch in length vertically on the surface of each lemon using a sharp knife.
Roll the lemon firmly on a counter to release some of the juices. Insert the one copper strip and one zinc strip vertically into the lemon, with one end sticking out. Connect one wire lead to each metal strip (electrode). Connect the remaining free end of the wire lead to the remaining free wire on the bulb.
This releases the juices inside and we want our lemons as juicy as possible. Start with one lemon and make a small cut through the peel on either end. It is very important that you place these far enough apart that the electrodes don't touch. Insert a copper plate on one side and a zinc plate on the other side.
Lemon Battery Science Fair Project. Colleen Beck OTR/L. February 2, 2023. Lemon STEM is such a fun way to explore concepts and this lemon battery science fair project is a winner! By using a lemon and a few other materials, you can discover the chemical process of moving electric current through a lemon to create a lemon battery. This lemon ...
Instructions: STEP 1: Line up your lemons. STEP 2: Place a nail in one end of each lemon. STEP 3: Cut a small slit at the other end of each lemon. Place a penny in each slit. STEP 4: Connect your clips to your lemons. Start with one clip on a nail and the other end unconnected.
Electrical circuit with lemons. A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb. We're going "Back To School ...
This week's demonstration is all about what to do when life hands you lemons
Add more lemon batteries connecting between electrodes with wires to increase the voltage enough to run an LED light, digital watch or basic calculator. Building a lemon battery is a good science experiment for home-schooled kids grades 3-8. Print off a lab sheet or experiment guide online such as one from the National Engineers Week Foundation.
Three varieties of lemon such as Kagoji, Sarboti and Elachi were used for the experiments. Elachi could produce maximum. 1.0±0.1 v voltage and 1.25±0.05 mA electricity. Overall, the electricity ...
Insert the copper and magnesium plates into one lemon half at a distance of about 1 cm apart from each other. Repeat with the other two plates using the other lemon half. Make sure that the plates do not touch each other. Take a light emitting diode. Connect a free end of the red crocodile clip wire to a long leg of the diode.
Cut off the bottom of each lemon so that it can sit upright. Then, carefully slice off the top to expose the flesh of the lemon inside. You can use a whole lemon or half of the lemon for this experiment. Loosen the Pulp: Use a craft stick or spoon to stir and mash the pulp inside the lemon, which helps release the citric acid in the lemon juice.