An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager

Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
A cellular mechanism of muscle memory facilitates mitochondrial remodelling following resistance training
Affiliations.
- 1 Department of Kinesiology, College of Public Health, Temple University, Philadelphia, PA, USA.
- 2 Cardiovascular Research Center, Lewis Katz School of Medicine, Temple University, Philadelphia, PA, USA.
- 3 School of Sports and Health Science, Kyungsung University, Busan, South Korea.
- 4 Mechanical & Molecular Myology Lab, Department of Rehabilitation Medicine and College of Medicine, Seoul National University, Bundang Hospital, Seongnam, South Korea.
- 5 School of Exercise and Sport Science, University of Ulsan, Ulsan, South Korea.
- 6 Center for Translational Medicine, Lewis Katz School of Medicine, Temple University, Philadelphia, PA, USA.
- 7 Department of Epidemiology and Biostatistics, College of Public Health, Temple University, Philadelphia, PA, USA.
- 8 School of Kinesiology, Auburn University, Auburn, AL, USA.
- 9 Department of Counseling, Health and Kinesiology, Texas A&M University-San Antonio, San Antonio, TX, USA.
- PMID: 30099751
- PMCID: PMC6138296
- DOI: 10.1113/JP275308
Key points: Referring to the muscle memory theory, previously trained muscles acquire strength and volume much faster than naive muscles. Using extreme experimental models such as synergist ablation or steroid administration, previous studies have demonstrated that the number of nuclei increases when a muscle becomes enlarged, which serves as a cellular muscle memory mechanism for the muscle. In the present study, we found that, when rats were subjected to physiologically relevant resistance training, the number of myonuclei increased and was retained during a long-term detraining period. The acquired myonuclei were related to a greater degree of muscle hypertrophic and mitochondrial biogenesis processes following subsequent hypertrophic conditions. Our data suggest a cellular mechanism supporting the notion that exposing young muscles to resistance training would help to restore age-related muscle loss coupled with mitochondrial dysfunction in later life.
Abstract: Muscle hypertrophy induced by resistance training is accompanied by an increase in the number of myonuclei. The acquired myonuclei are viewed as a cellular component of muscle memory by which muscle enlargement is promoted during a re-training period. In the present study, we investigated the effect of exercise preconditioning on mitochondrial remodelling induced by resistance training. Sprague-Dawley rats were divided into four groups: untrained control, training, pre-training or re-training. The training groups were subjected to weight loaded-ladder climbing exercise training. Myonuclear numbers were significantly greater (up to 20%) in all trained muscles compared to untrained controls. Muscle mass was significantly higher in the re-training group compared to the training group (∼2-fold increase). Mitochondrial content, mitochondrial biogenesis gene expression levels and mitochondrial DNA copy numbers were significantly higher in re-trained muscles compared to the others. Oxidative myofibres (type I) were significantly increased only in the re-trained muscles. Furthermore, in vitro studies using insulin-like growth factor-1-treated L6 rat myotubes demonstrated that myotubes with a higher myonuclear number confer greater expression levels of both mitochondrial and nuclear genes encoding for constitutive and regulatory mitochondrial proteins, which also showed a greater mitochondrial respiratory function. These data suggest that myonuclei acquired from previous training facilitate mitochondrial biogenesis in response to subsequent retraining by (at least in part) enhancing cross-talk between mitochondria and myonuclei in the pre-conditioned myofibres.
Keywords: mitochondrial biogenesis; muscle memory; myonuclei; resistance training.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
PubMed Disclaimer
Figure 1. Experimental design
Schematic diagram of…
Schematic diagram of the experimental design.
Figure 2. Pre‐trained myofibres present greater number…
Figure 2. Pre‐trained myofibres present greater number of myonuclei compared to untrained fibres
Figure 3. Muscle mass and muscle fibre…
Figure 3. Muscle mass and muscle fibre typing
Figure 4. Mitochondrial respiratory complex enzymatic activities
Figure 5. Previously trained muscle showed greater…
Figure 5. Previously trained muscle showed greater mitochondrial adaptations following re‐training compared to non‐pretrained muscles
Figure 6. Enhanced mitochondrial gene expression response…
Figure 6. Enhanced mitochondrial gene expression response in myonuclear‐enriched myotubes after AICAR treatment
Figure 7. Enhanced nuclear‐mitochondrial cross‐talk in pre‐trained…
Figure 7. Enhanced nuclear‐mitochondrial cross‐talk in pre‐trained skeletal muscle
Schematic diagram of the proposed mechanism…
- Muscle memory: virtues of your youth? Gundersen K, Bruusgaard JC, Egner IM, Eftestøl E, Bengtsen M. Gundersen K, et al. J Physiol. 2018 Sep;596(18):4289-4290. doi: 10.1113/JP276354. Epub 2018 Aug 25. J Physiol. 2018. PMID: 30145845 Free PMC article. No abstract available.
Similar articles
- Effects of training, detraining, and retraining on strength, hypertrophy, and myonuclear number in human skeletal muscle. Psilander N, Eftestøl E, Cumming KT, Juvkam I, Ekblom MM, Sunding K, Wernbom M, Holmberg HC, Ekblom B, Bruusgaard JC, Raastad T, Gundersen K. Psilander N, et al. J Appl Physiol (1985). 2019 Jun 1;126(6):1636-1645. doi: 10.1152/japplphysiol.00917.2018. Epub 2019 Apr 11. J Appl Physiol (1985). 2019. PMID: 30991013
- Muscle memory: myonuclear accretion, maintenance, morphology, and miRNA levels with training and detraining in adult mice. Murach KA, Mobley CB, Zdunek CJ, Frick KK, Jones SR, McCarthy JJ, Peterson CA, Dungan CM. Murach KA, et al. J Cachexia Sarcopenia Muscle. 2020 Dec;11(6):1705-1722. doi: 10.1002/jcsm.12617. Epub 2020 Sep 2. J Cachexia Sarcopenia Muscle. 2020. PMID: 32881361 Free PMC article.
- The effect of resistance training, detraining and retraining on muscle strength and power, myofibre size, satellite cells and myonuclei in older men. Blocquiaux S, Gorski T, Van Roie E, Ramaekers M, Van Thienen R, Nielens H, Delecluse C, De Bock K, Thomis M. Blocquiaux S, et al. Exp Gerontol. 2020 May;133:110860. doi: 10.1016/j.exger.2020.110860. Epub 2020 Feb 1. Exp Gerontol. 2020. PMID: 32017951
- Muscle memory and a new cellular model for muscle atrophy and hypertrophy. Gundersen K. Gundersen K. J Exp Biol. 2016 Jan;219(Pt 2):235-42. doi: 10.1242/jeb.124495. J Exp Biol. 2016. PMID: 26792335 Review.
- Myonuclear permanence in skeletal muscle memory: a systematic review and meta-analysis of human and animal studies. Rahmati M, McCarthy JJ, Malakoutinia F. Rahmati M, et al. J Cachexia Sarcopenia Muscle. 2022 Oct;13(5):2276-2297. doi: 10.1002/jcsm.13043. Epub 2022 Aug 12. J Cachexia Sarcopenia Muscle. 2022. PMID: 35961635 Free PMC article. Review.
- Resistance training diminishes mitochondrial adaptations to subsequent endurance training in healthy untrained men. Mesquita PHC, Godwin JS, Ruple BA, Sexton CL, McIntosh MC, Mueller BJ, Osburn SC, Mobley CB, Libardi CA, Young KC, Gladden LB, Roberts MD, Kavazis AN. Mesquita PHC, et al. J Physiol. 2023 Sep;601(17):3825-3846. doi: 10.1113/JP284822. Epub 2023 Jul 20. J Physiol. 2023. PMID: 37470322
- Resistance Training Diminishes Mitochondrial Adaptations to Subsequent Endurance Training. Mesquita PHC, Godwin JS, Ruple BA, Sexton CL, McIntosh MC, Mueller BJ, Osburn SC, Mobley CB, Libardi CA, Young KC, Gladden LB, Roberts MD, Kavazis AN. Mesquita PHC, et al. bioRxiv [Preprint]. 2023 Apr 6:2023.04.06.535919. doi: 10.1101/2023.04.06.535919. bioRxiv. 2023. Update in: J Physiol. 2023 Sep;601(17):3825-3846. doi: 10.1113/JP284822. PMID: 37066356 Free PMC article. Updated. Preprint.
- Daily Walking Accompanied with Intermittent Resistance Exercise Prevents Osteosarcopenia: A Large Cohort Study. Lee S, Kim JS, Park KS, Baek KW, Yoo JI. Lee S, et al. J Bone Metab. 2022 Nov;29(4):255-263. doi: 10.11005/jbm.2022.29.4.255. Epub 2022 Nov 30. J Bone Metab. 2022. PMID: 36529868 Free PMC article.
- Exercise sustains the hallmarks of health. Qiu Y, Fernández-García B, Lehmann HI, Li G, Kroemer G, López-Otín C, Xiao J. Qiu Y, et al. J Sport Health Sci. 2023 Jan;12(1):8-35. doi: 10.1016/j.jshs.2022.10.003. Epub 2022 Oct 29. J Sport Health Sci. 2023. PMID: 36374766 Free PMC article. Review.
- Elevated muscle mass accompanied by transcriptional and nuclear alterations several months following cessation of resistance-type training in rats. Rader EP, Baker BA. Rader EP, et al. Physiol Rep. 2022 Oct;10(20):e15476. doi: 10.14814/phy2.15476. Physiol Rep. 2022. PMID: 36259109 Free PMC article.
- Allen DL, Monke SR, Talmadge RJ, Roy RR & Edgerton VR. (1995). Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. J Appl Physiol 78, 1969–1976. - PubMed
- Amthor H, Otto A, Vulin A, Rochat A, Dumonceaux J, Garcia L, Mouisel E, Hourde C, Macharia R, Friedrichs M, Relaix F, Zammit PS, Matsakas A, Patel K & Partridge T. (2009). Muscle hypertrophy driven by myostatin blockade does not require stem/precursor‐cell activity. Proc Natl Acad Sci U S A 106, 7479–7484. - PMC - PubMed
- Barres R, Osler ME, Yan J, Rune A, Fritz T, Caidahl K, Krook A & Zierath JR. (2009). Non‐CpG methylation of the PGC‐1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab 10, 189–198. - PubMed
- Blaauw B, Canato M, Agatea L, Toniolo L, Mammucari C, Masiero E, Abraham R, Sandri M, Schiaffino S & Reggiani C. (2009). Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J 23, 3896–3905. - PubMed
- Bolster DR, Jefferson LS & Kimball SR. (2004). Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin‐, amino acid‐ and exercise‐induced signalling. Proc Nutr Soc 63, 351–356. - PubMed
Publication types
- Search in MeSH
Related information
Grants and funding.
- R01 HL126952/HL/NHLBI NIH HHS/United States
- NRF-2011-356-G00013/National Research Foundation of Korea (NRF)/International
- 12SDG12070327/American Heart Association (AHA)/International
- R01HL126952/HHS | National Institutes of Health (NIH)/International
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
- PubMed Central
Other Literature Sources
- scite Smart Citations

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- DOI: 10.1242/jeb.124495
- Corpus ID: 18707695
Muscle memory and a new cellular model for muscle atrophy and hypertrophy
- K. Gundersen
- Published in Journal of Experimental… 1 January 2016
- Biology, Medicine
Figures from this paper

125 Citations
The concept of skeletal muscle memory: evidence from animal and human studies, skeletal muscles do not undergo apoptosis during either atrophy or programmed cell death-revisiting the myonuclear domain hypothesis.
- Highly Influenced
Satellite cell depletion prevents fiber hypertrophy in skeletal muscle
Myonuclear permanence in skeletal muscle memory: a systematic review and meta‐analysis of human and animal studies, transcriptional memory in skeletal muscle. don't forget (to) exercise, skeletal muscle fibers count on nuclear numbers for growth., role of damage and management in muscle hypertrophy: different behaviors of muscle stem cells in regeneration and hypertrophy., molecular and cellular aspects of sarcopenia, muscle healthy aging and physical conditioning in the elderly, elevated myonuclear density during skeletal muscle hypertrophy in response to training is reversed during detraining., a juvenile climbing exercise establishes a muscle memory boosting the effects of exercise in adult rats, 117 references, a cellular memory mechanism aids overload hypertrophy in muscle long after an episodic exposure to anabolic steroids.
- Highly Influential
Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining
- 11 Excerpts
Myonuclear domains in muscle adaptation and disease
Nuclear domains during muscle atrophy: nuclei lost or paradigm lost, muscle hypertrophy induced by the ski protein: cyto-architecture and ultrastructure., the behaviour of satellite cells in response to exercise: what have we learned from human studies, a regenerative change during muscle adaptation to denervation in rats., muscle hypertrophy driven by myostatin blockade does not require stem/precursor-cell activity, no change in myonuclear number during muscle unloading and reloading., potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis., related papers.
Showing 1 through 3 of 0 Related Papers
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 15 August 2022
Fitness tracking reveals task-specific associations between memory, mental health, and physical activity
- Jeremy R. Manning 1 ,
- Gina M. Notaro 1 ,
- Esme Chen 1 &
- Paxton C. Fitzpatrick 1
Scientific Reports volume 12 , Article number: 13822 ( 2022 ) Cite this article
35k Accesses
3 Citations
596 Altmetric
Metrics details
- Cognitive neuroscience
- Human behaviour
Physical activity can benefit both physical and mental well-being. Different forms of exercise (e.g., aerobic versus anaerobic; running versus walking, swimming, or yoga; high-intensity interval training versus endurance workouts; etc.) impact physical fitness in different ways. For example, running may substantially impact leg and heart strength but only moderately impact arm strength. We hypothesized that the mental benefits of physical activity might be similarly differentiated. We focused specifically on how different intensities of physical activity might relate to different aspects of memory and mental health. To test our hypothesis, we collected (in aggregate) roughly a century’s worth of fitness data. We then asked participants to fill out surveys asking them to self-report on different aspects of their mental health. We also asked participants to engage in a battery of memory tasks that tested their short and long term episodic, semantic, and spatial memory performance. We found that participants with similar physical activity habits and fitness profiles tended to also exhibit similar mental health and task performance profiles. These effects were task-specific in that different physical activity patterns or fitness characteristics varied with different aspects of memory, on different tasks. Taken together, these findings provide foundational work for designing physical activity interventions that target specific components of cognitive performance and mental health by leveraging low-cost fitness tracking devices.
Similar content being viewed by others

Cross-sectional and longitudinal neural predictors of physical activity and sedentary behaviour from a 6-month randomized controlled trial

Associations of timing of physical activity with all-cause and cause-specific mortality in a prospective cohort study
An umbrella review of randomized control trials on the effects of physical exercise on cognition
Introduction.
Engaging in physical activity (exercise) can improve physical fitness by increasing muscle strength 1 , 2 , 3 , 4 , bone density 5 , 6 , 7 , cardiovascular performance 8 , 9 , lung capacity 10 (although see 11 ), and endurance 12 . Physical activity can also improve mental health 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 and cognitive performance 19 , 24 , 25 , 26 .
The physical benefits of exercise can be explained by stress-responses of the affected body tissues. For example, skeletal muscles that are taxed during exercise exhibit stress responses 27 that can in turn affect their growth or atrophy 28 . By comparison, the benefits of physical activity on mental health are less direct. For example, one hypothesis is that physical activity leads to specific physiological changes, such as increased aminergic synaptic transmission and endorphin release, which in turn act on neurotransmitters in the brain 18 . Speculatively, if different physical activity regimens lead to different neurophysiological responses, one might be able to map out a spectrum of signalling and transduction pathways that are impacted by a given type, duration, and intensity of physical activity in each brain region. For example, prior work has shown that physical activity increases acetylcholine levels, starting in the vicinity of the exercised muscles 29 . Acetylcholine is thought to play an important role in memory formation (e.g., by modulating specific synaptic inputs from entorhinal cortex to the hippocampus, albeit in rodents 30 ). Given the central role that these medial temporal lobe structures play in memory, changes in acetylcholine might lead to specific changes in memory formation and retrieval.
In the present study, we hypothesize that (a) different intensities of physical activity will have different quantifiable impacts on cognitive performance and mental health, and that (b) these impacts will be consistent across individuals. To this end, we collected a year of real-world fitness tracking data from each of 113 participants. We then asked each participant to fill out a brief survey in which they self-evaluated and self-reported several aspects of their mental health. Finally, we ran each participant through a battery of memory tasks, which we used to evaluate their memory performance along several dimensions. We searched the data for potential associations between memory, mental health, and physical activity.
We ran an online experiment using the Amazon Mechanical Turk (MTurk) platform 31 . We collected data about each participant’s fitness and physical activity habits, a variety of self-reported measures concerning their mental health, and about their performance on a battery of memory tasks.
Participants
We recruited experimental participants by posting our experiment as a Human Intelligence Task (HIT) on the MTurk platform. We limited participation to MTurk Workers who had been assigned a “master worker” designation on the platform, given to workers who score highly across several metrics on a large number of HITs, according to a proprietary algorithm managed by Amazon. One criterion embedded into the algorithm is a requirement that master workers must maintain a HIT acceptance rate of at least 95%. We further limited our participant pool to participants who self-reported that they were fluent in English and regularly used a Fitbit fitness tracker device. A total of 160 workers accepted our HIT in order to participate in our experiment. Of these, we excluded all participants who failed to log into their Fitbit account (giving us access to their anonymized fitness tracking data), encountered technical issues (e.g., by accessing the HIT using an incompatible browser, device, or operating system), or who ended their participation prematurely, before completing the full study. In all, 113 participants contributed usable data to the study.
For their participation, workers received a base payment of $5 per hour (computed in 15 min increments, rounded up to the nearest 15 min), plus an additional performance-based bonus of up to $5. Our recruitment procedure and study protocol were approved by Dartmouth’s Committee for the Protection of Human Subjects. We obtained informed consent using an online form administered to all prospective participants prior to enrolling them in our study. All methods were performed in accordance with the relevant guidelines and regulations.
Gender, age, and race
Of the 113 participants who contributed usable data, 77 reported their gender as female, 35 as male, and 1 chose not to report their gender. Participants ranged in age from 19 to 68 years old (25th percentile: 28.25 years; 50th percentile: 32 years; 75th percentile: 38 years). Participants reported their race as White (90 participants), Black or African American (11 participants), Asian (7 participants), Other (4 participants), and American Indian or Alaska Native (3 participants). One participant opted not to report their race.
All participants reported that they were fluent in either 1 or 2 languages (25th percentile: 1; 50th percentile: 1; 75th percentile: 1), and that they were “familiar” with between 1 and 11 languages (25th percentile: 1; 50th percentile: 2; 75th percentile: 3).
Reported medical conditions and medications
Participants reported having and/or taking medications pertaining to the following medical conditions: anxiety or depression (4 participants), recent head injury (2 participants), high blood pressure (1 participant), bipolar disorder (1 participant), hypothyroidism (1 participant), and other unspecified conditions or medications (1 participant). Participants reported their current and typical stress levels on a Likert scale as very relaxed (− 2), a little relaxed (− 1), neutral (0), a little stressed (1), or very stressed (2). The “current” stress level reflected participants’ stress at the time they participated in the experiment. Their responses ranged from − 2 to 2 (current stress: 25th percentile: − 2; 50th percentile: − 1; 75th percentile: 1; typical stress: 25th percentile: 0; 50th percentile: 1; 75th percentile: 1). Participants also reported their current level of alertness on a Likert scale as very sluggish (− 2), a little sluggish (− 1), neutral (0), a little alert (1), or very alert (2). Their responses ranged from − 2 to 2 (25th percentile: 0; 50th percentile: 1; 75th percentile: 2). Nearly all (111 out of 113) participants reported that they had normal color vision, and 15 participants reported uncorrected visual impairments (including dyslexia and uncorrected near- or far-sightedness).
Residence and level of education
Participants reported their residence as being located in the suburbs (36 participants), a large city (30 participants), a small city (23 participants), rural (14 participants), or a small town (10 participants). Participants reported their level of education as follows: College graduate (42 participants), Master’s degree (23 participants), Some college (21 participants), High school graduate (9 participants), Associate’s degree (8 participants), Other graduate or professional school (5 participants), Some graduate training (3 participants), or Doctorate (2 participants).
Reported water and coffee intake
Participants reported the number of 8 oz cups of water and coffee they had consumed prior to accepting the HIT. Water consumption ranged from 0 to 6 cups (25th percentile: 1; 50th percentile: 3; 75th percentile: 4). Coffee consumption ranged from 0 to 4 cups (25th percentile: 0; 50th percentile: 1; 75th percentile: 2).
Upon accepting the HIT posted on MTurk, each worker was directed to read and fill out a screening and consent form, and to share access to their anonymized Fitbit data via their Fitbit account. After consenting to participate in our study and successfully sharing their Fitbit data, participants filled out a survey and then engaged in a series of memory tasks (Fig. 1 ). All stimuli and code for running the full MTurk experiment may be found at https://github.com/ContextLab/brainfit-task .
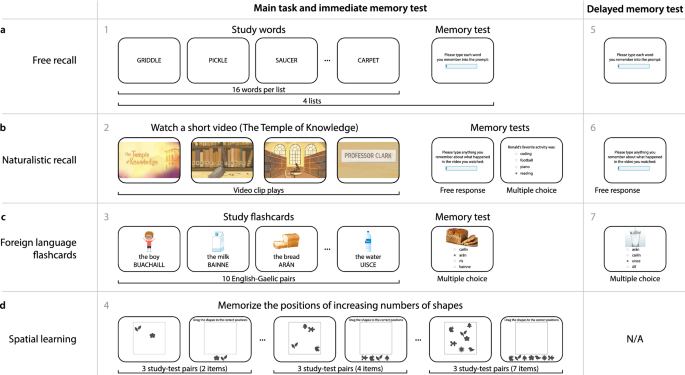
Battery of memory tasks. ( a ) Free recall. Participants study 16 words (presented one at a time), followed by an immediate memory test where they type each word they remember from the just-studied list. In the delayed memory test, participants type any words they remember studying, from any list. ( b ) Naturalistic recall. Participants watch a brief video, followed by two immediate memory tests. The first test asks participants to write out what happened in the video. The second test has participants answer a series of multiple choice questions about the conceptual content of the video. In the delayed memory test, participants (again) write out what happened in the video. ( c ) Foreign language flashcards. Participants study a sequence of 10 English-Gaelic word pairs, each presented with an illustration of the given word. During an immediate memory test, participants perform a multiple choice test where they select the Gaelic word that corresponds to the given photograph. During the delayed memory test, participants perform a second multiple choice test, where they select the Gaelic word that corresponds to each of a new set of photographs. ( d ) Spatial learning. In each trial, participants study a set of randomly positioned shapes. Next, the shapes’ positions are altered, and participants are asked to drag the shapes back to their previous positions. All panels. The gray numbers denote the order in which participants experienced each task or test.
Survey questions
We collected the following demographic information from each participant: their birth year, gender, highest (academic) degree achieved, race, language fluency, and language familiarity. We also collected information about participants’ health and wellness, including about their vision, alertness, stress, sleep, coffee and water consumption, location of their residence, activity typically required for their job, and physical activity habits.
Free recall (Fig. 1 a)
Participants studied a sequence of four word lists, each comprising 16 words. After studying each list, participants received an immediate memory test, whereby they were asked to type (one word at a time) any words they remembered from the just-studied list, in any order.
Words were presented for 2 s each, in black text on a white background, followed by a 2 s blank (white) screen. After the final 2 s pause, participants were given 90 s to type in as many words as they could remember, in any order. The memory test was constructed such that the participant could only see the text of the current word they were typing; when they pressed any non-letter key, the current word was submitted and the text box they were typing in was cleared. This was intended to prevent participants from retroactively editing their previous responses.
The word lists participants studied were drawn from the categorized lists reported by 32 . Each participant was assigned four unique randomly chosen lists (in a randomized order), selected from a full set of 16 lists. Each chosen list was then randomly shuffled before presenting the words to the participants. Participants also performed a final delayed memory test where they were given 180 s to type out any words they remembered from any of the 4 lists they had studied.
Recalled words within an edit distance of 2 (i.e., a Levenshtein Distance less than or equal to 2) of any word in the wordpool were “autocorrected” to their nearest match. We also manually corrected clear typos or misspellings by hand (e.g., we corrected “hippoptumas” to “hippopotamus”, “zucinni” to “zucchini”, and so on). Finally, we lemmatized each submitted word to match the plurality of the matching wordpool word (e.g., “bongo” was corrected to “bongos”, and so on). After applying these corrections, any submitted words that matched words presented on the just-studied list were tagged as “correct” recalls, and any non-matching words were discarded as “errors.” Because participants were not allowed to edit the text they entered, we chose not to analyze these putative “errors,” since we could not distinguish typos from true misrememberings.
Naturalistic recall (Fig. 1 b)
Participants watched a 2.5-min video clip entitled “The Temple of Knowledge.” The video comprises an animated story told to StoryCorps by Ronald Clark, who was interviewed by his daughter, Jamilah Clark. The narrator (Ronald) discusses growing up living in an apartment over the Washington Heights branch of the New York Public Library, where his father worked as a custodian during the 1940s.
After watching the video clip, participants were asked to type out anything they remembered about what happened in the video. They typed their responses into a text box, one sentence at a time. When the participant pressed the return key or typed any final punctuation mark (“.”, “!”, or “?”) the text currently entered into the box was “submitted” and added to their transcript, and the text box was cleared to prevent further editing of any already-submitted text. This was intended to prevent participants from retroactively editing their previous responses. Participants were given up to 10 min to enter their responses. After 4 min, participants were given the option of ending the response period early, e.g., if they felt they had finished entering all the information they remembered. Each participant’s transcript was constructed from their submitted responses by combining the sentences into a single document and removing extraneous whitespace characters. Following this 4–10-min free response period, participants were given a series of 10 multiple choice questions about the conceptual content of the story. All participants received the same questions, in the same order. Participants also performed a final delayed memory test, where they carried out the free response recall task a second time, near the end of the testing session. This resulted in a second transcript, for each participant.
Foreign language flashcards (Fig. 1 c)
Participants studied a series of 10 English-Gaelic word pairs in a randomized order. We selected the Gaelic language both for its relatively small number of native speakers and for its dissimilarity to other commonly spoken languages amongst MTurk workers. We verified (via self report) that all of our participants were fluent in English and that they were neither fluent nor familiar with Gaelic.
Each word’s “flashcard” comprised a cartoon depicting the given word, the English word or phrase in lowercase text (e.g., “the boy”), and the Gaelic word or phrase in uppercase text (e.g., “BUACHAILL”). Each flashcard was displayed for 4 s, followed by a 3 s interval (during which the screen was cleared) prior to the next flashcard presentation.
After studying all 10 flashcards, participants were given a multiple choice memory test where they were shown a series of novel photographs, each depicting one of the 10 words they had learned. They were asked to select which (of 4 unique options) Gaelic word went with the given picture. The 3 incorrect options were selected at random (with replacement across trials), and the orders in which the choices appeared to the participant were also randomized. Each of the 10 words they had learned was tested exactly once.
Participants also performed a final delayed memory test, where they were given a second set of 10 questions (again, one per word they had studied). For this second set of questions participants were prompted with a new set of novel photographs, and new randomly chosen incorrect choices for each question. Each of the 10 original words they had learned were (again) tested exactly once during this final memory test.
Spatial learning (Fig. 1 d)
Participants performed a series of study-test trials where they memorized the onscreen spatial locations of two or more shapes. During the study phrase of each trial, a set of shapes appeared on the screen for 10 s, followed by 2 s of blank (white) screen. During the test phase of each trial, the same shapes appeared onscreen again, but this time they were vertically aligned and sorted horizontally in a random order. Participants were instructed to drag (using the mouse) each shape to its studied position, and then to click a button to indicate that the placements were complete.
In different study-test trials, participants learned the locations of different numbers of shapes (always drawn from the same pool of 7 unique shapes, where each shape appeared at most one time per trial). They first performed three trials where they learned the locations of 2 shapes; next three trials where they learned the locations of 3 shapes; and so on until their last three trials, where (during each trial) they learned the locations of 7 shapes. All told, each participant performed 18 study-test trials of this spatial learning task (3 trials for each of 2, 3, 4, 5, 6, and 7 shapes).
Fitness tracking using Fitbit devices
To gain access to our study, participants provided us with access to all data associated with their Fitbit account from the year (365 calendar days) up to and including the day they accepted the HIT. We filtered out all identifiable information (e.g., participant names, GPS coordinates, etc.) prior to importing their data.
Collecting and processing Fitbit data
The fitness tracking data associated with participants’ Fitbit accounts varied in scope and duration according to which device the participant owned (Fig. S1 ), how often the participant wore (and/or synced) their tracking device, and how long they had owned their device. For example, while all participants’ devices supported basic activity metrics such as daily step counts, only a subset of the devices with heart rate monitoring capabilities provided information about workout intensity, resting heart rate, and other related measures. Across all devices, we collected the following information: heart rate data, sleep tracking data, logged bodyweight measurements, logged nutrition measurements, Fitbit account and device settings, and activity metrics.
If available, we extracted all heart rate data collected by participants’ Fitbit device(s) and associated with their Fitbit profile. Depending on the specific device model(s) and settings, this included second-by-second, minute-by-minute, daily summary, weekly summary, and/or monthly summary heart rate information. These summaries include information about participants’ average heart rates, and the amount of time they were estimated to have spent in different “heart rate zones” (rest, out-of-range, fat burn, cardio, or peak, as defined by their Fitbit profile), as well as an estimate of the number of estimated calories burned while in each heart rate zone.
If available, we extracted all sleep data collected by participants’ Fitbit device(s). Depending on the specific device model(s) and settings, this included nightly estimates of the duration and quality of sleep, as well as the amount of time spent in each sleep stage (awake, REM, light, or deep).
If available, we extracted any weight-related information affiliated with participants’ Fitbit accounts within 1 year prior to enrolling in our study. Depending on their specific device model(s) and settings, this included their weight, body mass index, and/or body fat percentage.
If available, we extracted any nutrition-related information affiliated with participants’ Fitbit accounts within 1 year prior to enrolling in our study. Depending on their specific account settings and usage behaviors, this included a log of the specific foods they had eaten (and logged) over the past year, and the amount of water consumed (and logged) each day.
Account and device settings
We extracted any settings associated with participants’ Fitbit accounts to determine (a) which device(s) and model(s) are associated with their Fitbit account, (b) time(s) when their device(s) were last synced, and (c) battery level(s).
Activity metrics
If available, we extracted any activity-related information affiliated with participants’ Fitbit accounts within 1 year prior to enrolling in our study. Depending on their specific device model(s) and settings, this included: daily step counts; daily amount of time spent in each activity level (sedentary, lightly active, fairly active, or very active, as defined by their account settings and preferences); daily number of floors climbed; daily elevation change; and daily total distance traveled.
Comparing recent versus baseline measurements.
We were interested in separating out potential associations between absolute fitness metrics and relative metrics. To this end, in addition to assessing potential raw (absolute) fitness metrics, we also defined a simple measure of recent changes in those metrics, relative to a baseline:
where m ( i ) is the value of metric m from \(i - 1\) days prior to testing (e.g., m (1) represents the value of m on the day the participant accepted the HIT, and m (10) represents the value of m 9 days prior to accepting the HIT). We set \(R = 7\) and \(B = 30\) . In other words, to estimate recent changes in any metric m , we divided the average value of m taken over the prior week by the average value of m taken over the 30 days before that.
Exploratory correlation analyses
We used a bootstrap procedure to identify reliable correlations between different memory-related, fitness-related, and demographic-related variables. For each of \(N = 10,000\) iterations, we selected (with replacement) a sample of 113 participants to include. This yielded, for each iteration, a sampled “data matrix” with one row per sampled participant and one column for each measured variable. When participants were sampled multiple times in a given iteration, as was often the case, this matrix contained duplicate rows. Next, we computed the Pearson’s correlation between each pair of columns. This yielded, for each pair of columns, a distribution of N bootstrapped correlation coefficients. If \(97.5\%\) or fewer of the coefficients for a given pair of columns had the same sign, we excluded the pair from further analysis and considered the expected correlation between those columns to be undefined. If \(> 97.5\%\) of the coefficients for a given pair of columns had the same sign (corresponding to a bootstrap-estimated two-tailed p threshold of 0.05), we computed the expected correlation coefficient as:
where \(m(x)_n\) represents column x of the bootstrapped data matrix for iteration n , \(\tanh\) is the hyperbolic tangent, and \(\tanh ^{-1}\) is the inverse hyperbolic tangent. We estimated the corresponding p -values for these correlations as one minus the proportion of bootstrapped correlations with the same sign, multiplied by two.
Reverse correlation analyses
We sought to characterize potential associations between the dynamics of participants’ fitness-related activities leading up to the time they participated in a memory task and their performance on the given task. For each fitness-related variable, we constructed a timeseries matrix whose rows corresponded to timepoints (sampled once per day) leading up to the day the participant accepted the HIT for our study, and whose columns corresponded to different participants. These matrices often contained missing entries, since different participants’ Fitbit devices tracked fitness-related activities differently. For example, participants whose Fitbit devices lacked heart rate sensors would have missing entries for any heart rate-related variables. Or, if a given participant neglected to wear their fitness tracker on a particular day, the column corresponding to that participant would have missing entries for that day. To create stable estimates, we smoothed the timeseries of each fitness measure using a sliding window of 1 week. In other words, for each fitness measure, we replaced the “observed value” for each day with the average values of that measure (when available) over the 7-day interval ending on the given day.
In addition to this set of matrices storing timeseries data for each fitness-related variable, we also constructed a memory performance matrix, M , whose rows corresponded to different memory-related variables, and whose columns corresponded to different participants. For example, one row of the memory performance matrix reflected the average proportion of words (across lists) that each participant remembered during the immediate free recall test, and so on.
Given a fitness timeseries matrix, F , we computed the weighted average and weighted standard error of the mean of each row of F , where the weights were given by a particular memory-related variable (row of M ). For example, if F contained participants’ daily step counts, we could use any row of M to compute a weighted average across any participants who contributed step count data on each day. Choosing a row of M that corresponded to participants’ performance on the naturalistic recall task would mean that participants who performed better on the naturalistic recall task would contribute more to the weighted average timeseries of daily step counts. Specifically, for each row, t , of F , we computed the weighted average (across the S participants) as:
where \({\dot{m}}\) denotes the normalized min-max scaling of m (the row of M corresponding to the chosen memory-related variable):
Here, \(\epsilon\) provides a lower bound on the influence of the lowest-weighted participant’s data. We defined \(\epsilon = 0.001\) , ensuring that the lowest-weighted participant had relatively low (but non-zero) influence. We computed the weighted standard error of the mean as:
When a given row of F was missing data from one or more participants, those participants were excluded from the weighted average for the corresponding timepoint and the weights (across all remaining participants) were re-normalized to sum to 1. The above procedure yielded, for each memory variable, a timeseries of weighted average (and weighted standard error of the mean) fitness tracking values leading up to the day of the experiment.
Before testing our main hypotheses, we examined the behavioral data from each of four memory tasks (Fig. 1 ): a random word list learning “free recall” task; a naturalistic recall task whereby participants watched a short video and then recounted the narrative; a foreign language “flashcards” task; and a spatial learning task. Each of the first three tasks (free recall, naturalistic recall, and the flashcards task) included both an immediate (short term) memory test and a delayed (long term) memory test. The spatial learning task included only an immediate test. Participants in all four tasks exhibited general trends and tendencies that have been previously reported in prior work. We were also interested in characterizing the variability in task performance across participants. For example, if all participants exhibited near-identical behaviors or performance on a given task, we would be unable to identify how memory performance on that task varied with mental health or physical activity.
When participants engaged in free recall of random word lists, they displayed strong primacy and recency effects 33 on the immediate memory tests (as reflected by improved memory for early and late list items; Fig. 2 a, left and right panels). On the delayed memory test, the recency effect was substantially diminished (Fig. 3 a, left and right panels), consistent with myriad previous studies (for review see 34 ). Participants also tended to cluster their recalls according to the words’ study positions 35 on both the immediate (Fig. 2 a, middle panel) and delayed (Fig. 3 a, middle panel) memory tests.
When participants engaged in naturalistic recall by recounting the narrative of a short story video, they reliably and accurately remembered the major narrative events on both the immediate (Fig. 2 b) and delayed (Fig. 3 b) tests. This is consistent with prior work showing that memory for rich narratives is both detailed and accurate 36 , 37 .
Performance on the foreign language flashcards task (immediate: Fig. 2 c; delayed: Fig. 3 c) varied substantially across participants, and did not show any clear serial position effects. Participants also displayed substantial variation in performance on the spatial learning task (Fig. 2 d). In general, participants reported the shape’s positions more accurately when there were fewer shapes. However, both the baseline estimation accuracy and the rate of decrease in accuracy as a function of increasing number of memorized locations varied substantially across participants.
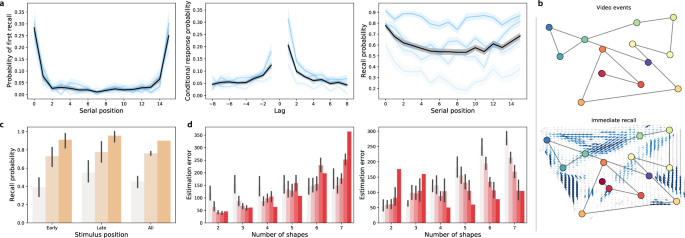
Immediate memory tests. ( a ) Free recall. Left: probability of recalling each word first as a function of its presentation position. Middle: probability of transitioning between successively recalling the word presented at position i , followed by word presented at position \(i + {\mathrm {Lag}}\) . Right: probability of recalling each word as a function of its presentation position. See Fig. S2 for additional details. ( b ) Naturalistic recall. Top: 2D embedding of a 2.5-min video clip; each dot reflects a narrative event (red denotes early events and blue denotes later events). Bottom: 2D embedding of the averaged transcripts of participants’ recountings of the narrative (dots: same format as top panel). The arrows denote the average trajectory directions through the corresponding region of text embedding space, for any participants whose recountings passed through that region. Blue arrows denote statistically reliable agreement across participants ( \(p < 0.05\) , corrected). See Fig. S3 for additional details. ( c ) Foreign language flashcards. Each bar denotes the average proportion of correctly recalled Gaelic-English word pairs from early (first 3), late (last 3), or all (i.e., all 10) study positions. See Fig. S4 for additional details. ( d ) Spatial learning. Average estimation error in shape locations as a function of the number of shapes. See Fig. S5 for additional details. All panels: error bars and error ribbons denote bootstrap-estimated 95% confidence intervals. Shading (saturation) denotes results for different subsets of participants assigned based on their task performance (Figs. S2 , S3 , S4 , and S5 provide information about which performance metrics and values the shading reflects; in general more saturated colors denote participants who performed better on the given task.) In panel d , participants are grouped in two ways; in the left panel, participants are grouped according to the y -intercepts of regression lines (estimation error as a function of the number of shapes); in the right panel, participants are grouped according to the slopes of the same regression lines.
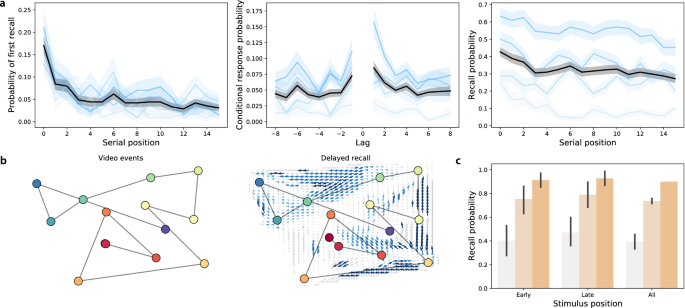
Delayed memory tests. ( a ) Free recall. These panels are in the same format as Fig. 2 a, but they reflect performance on the delayed free recall task. For additional details see Fig. S2 . ( b ) Naturalistic recall. These panels are in the same format as Fig. 2 b, but the right panel reflects performance on the delayed naturalistic recall task. For additional details see Fig. S3 . ( c ) Foreign language flashcards. This panel is in the same format as Fig. 2 c, but it reflects performance on the delayed flashcards test. For additional details see Fig. S4 .
In addition to observing substantial across-participant variability in memory performance, we also observed substantial variability in participants’ fitness and activity metrics (Fig. 4 ). We examined recent measurements, averaged over the week prior to testing (Fig. 4 a), baselined measurements (average over the prior week, divided by the average over the preceding 30 days; Fig. 4 b), along with more gradually varying measures that tended to remain relatively static over timescales of weeks to months (Fig. 4 c). Figure S6 displays across-participant distributions for a broad selection of these measures, and Figs. S7 , S8 , S9 , and S10 show different participants’ fitness metrics, broken down by their performance on different memory tasks.
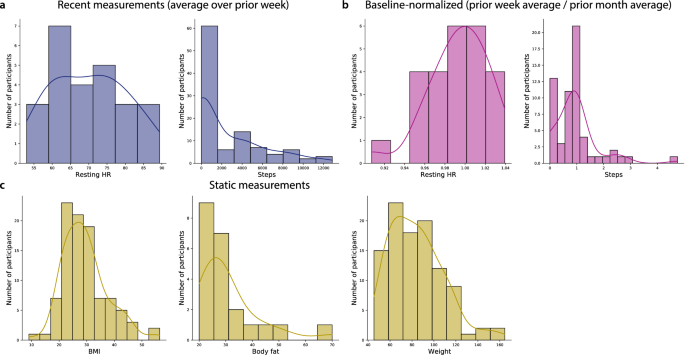
Fitness measures. ( a ) Recent measures. Resting heart rate (HR) and daily step counts, averaged over the week prior to testing. ( b ) Baseline-normalized measures. Resting heart rate and daily step counts averaged over the week prior to testing, divided by the average resting heart rate and step counts averaged over the preceding month. ( c ) Static measures. Body mass index (BMI), body fat percentage, and weight (in kg). For more information see Figs. S6 , S7 , S8 , S9 , and S10 .
We wondered about potential links between the different aspects of participants’ data. For example, if people who engaged in particular intensities of physical activity also tended to perform better on a given memory task, this could suggest that either (a) some property intrinsic to participants who exercised in a particular way might also affect their memory performance on the given task, and/or (b) particular physical activity behaviors could have a causal impact on memory performance. We carried out an exploratory analysis whereby we used a bootstrap-based approach to identify reliable correlations between different aspects of memory performance (Fig. S11 ), different aspects of fitness (Fig. S12 ), different demographic attributes (Fig. S13 ), and correlations between memory performance, fitness information, and demographic attributes (Fig. S14 ). Specifically, we sought to identify correlations that were present in the same direction (i.e., positive or negative) across different subsets of participants. For each test, we report the average correlation (taken across 10,000 subsets of participants, chosen with replacement) and an associated two-tailed p -value, estimated as
where q is the proportion of those 10,000 subsets that exhibited correlations in the same direction (see “ Exploratory correlation analyses ”). When all 10,000 randomly chosen subsets of participants exhibited correlations in the same direction (i.e., all positive correlations or all negative correlations), we report the p -value as \(p < 0.0001\) .
Several patterns emerged from these analyses. First, we found that participants’ performance on the (within-task) immediate versus delayed memory tests from the free recall, naturalistic recall, and foreign language flashcards tasks were positively correlated ( r s \(> 0.25\) , p s \(< 0.003\) ). This suggests that, within each of these tasks, similar processes or constraints may influence both short term and long term information retrieval. We also found reliable across-task correlations between participants’ (immediate and delayed) performance on the free recall and foreign language flashcards tasks ( r s \(> 0.3\) , p s \(< 0.03\) ).
A large number of fitness-related measures displayed reliable correlations (for a complete report, see Fig. S12 ). For example, body mass index (BMI) and weight were correlated ( \(r = 0.91,\,\, p < 0.0001\) ). Resting heart rate over the prior week was negatively correlated with recent low-to-moderate-intensity (“fat burn”) cardiovascular activity levels ( \(r = 0.70,\,\, p = 0.0004\) ). Participants’ peak heart rates (averaged over the prior week) were also negatively correlated with recent increases in step counts and daily elevation gains ( \(r{\mathrm {s}}< -0.26,\,\, p{\mathrm {s}} < 0.03\) ), where recent changes were defined as the average values over the 7 days leading up to the test day divided by the average values over the preceding 30 days. Several demographic attributes (Fig. S13 ) displayed trivial correlations (e.g., participants identifying as male never reported identifying as female, and so on). We also observed a negative correlation between reported stress and alertness ( \(r = -0.44,\,\, p < 0.0001\) ), and positive correlations between the reported clarity of the instructions for all tasks ( \(r{\mathrm {s}} > 0.26,\,\, p{\mathrm {s}} < 0.02\) ).
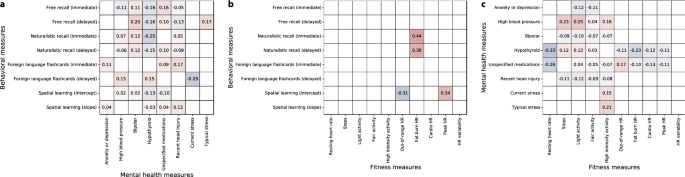
Summaries of correlations between behavioral, fitness, and mental health measures. The reported values in the tables reflect correlations between each pair of measures. Only statistically reliable correlations ( \(p < 0.05\) , corrected) are displayed. ( a ) Correlations between behavioral and mental health measures. We adjusted each task’s behavioral measure(s) such that more positive values reflect better performance on the given task. We used participants’ mean recall accuracies to characterize performance on the free recall and foreign language flashcards tasks, and mean precisions to characterize performance on the naturalistic recall tasks. We characterized performance on the spatial learning task using the (inverted and normalized) intercepts and slopes of linear regressions on mean estimation errors as a function of the numbers of studied shapes (also see Figs. 2 , 3 , S2 , S3 , S4 , and S5 ). For each mental health measure, more positive values denote greater severity of the given measure. Typical and current stress levels were measured by self report. Mental health information was inferred using each participants’ list of self-reported medications (see “ Methods ”). Positive correlations indicate that better performance on a given behavioral task is associated with more severe mental health phenotypes. ( b ) Correlations between fitness and mental health measures. For each fitness measure, more positive values denote higher observed scores (i.e., higher resting heart rate, more minutes of activity or time spent in each heart rate zone, or greater heart rate variability). The mental health measures in this panel were treated as in a . ( c ) Correlations between fitness and behavioral measures. Each measure reflected in this panel was treated as in panels a and b .
We also found reliable correlations between participants’ fitness and demographic measures and their behaviors in different tasks (Fig. 5 ; for a complete report, see Fig. S14 ). For example, recent low-to-moderate-intensity (“fat burn”) cardiovascular activity was positively correlated with immediate ( \(r = 0.44,\,\, p = 0.001\) ) and delayed ( \(r = 0.38,\,\, p = 0.031\) ) recall performance on the naturalistic memory task. Recent sedentary (“out-of-range”) cardiovascular activity was negatively correlated with performance on the spatial learning task ( \(r = -0.31,\,\, p = 0.042\) ), whereas recent high intensity (“peak”) activity was positively correlated with performance on the spatial learning task ( \(r = 0.34,\,\, p = 0.0002\) ). Mental health indicators, such as self-reported stress levels and medications were also associated with differences in memory (Fig. 5 a, Fig. S14 ). For example, self-reported stress levels at the time of test were negatively correlated with performance on the delayed memory test for the foreign language flashcards task ( \(r = -0.29,\,\, p = 0.038\) ), whereas participants who were medicated for anxiety and depression tended to perform slightly (but reliably) better on the immediate memory test for the foreign language flashcards task ( \(r = 0.11,\,\, p < 0.0001\) ). Mental health indicators were also correlated with several fitness measures (Fig. 5 c). For example, participants with higher resting heart rates were less likely to be hypothyroid ( \(r = -0.33,\,\, p < 0.0001\) ). Participants who engaged in more low-intensity (“light”) activity tended to be less anxious and depressed ( \(r = -0.12,\,\, p = 0.03\) ), whereas participants who engaged in more high-intensity activity tended to report higher levels of current ( \(r = 0.15,\,\, p = 0.027\) ) and typical ( \(r = 0.21,\,\, p = 0.012\) ) stress.
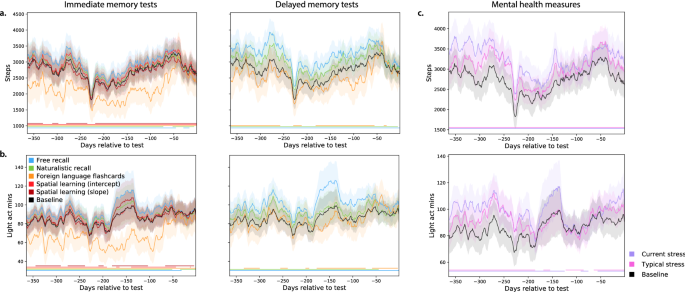
Dynamics of physical activity vary with memory performance and mental health measures. ( a ) Daily step counts. Each timecourse is weighted by either performance on immediate recall tests (left panel) or on delayed recall tests (right panel). The black (baseline) timecourses display the (unweighted) average across all participants. ( b ) Daily duration (in minutes) of low-intensity physical activity. Timecourses are displayed in the same format and color scheme as those in panel a . Analogous timecourses for additional fitness-related measures may be found in Figs. S15 , S16 , and S17 . ( c ) Timecourses of physical activity, weighted by mental health measures. The timecourses in each panel display the average daily step counts (top panel) or duration of low-intensity activity (bottom panel). The colored lines show average activity dynamics weighted by self-reported stress levels at the start of the experiment (purple) and self-reported “typical” stress levels (pink). The baseline curves (black) display the average across all participants (re-plotted in panel c to illustrate scale differences across panels). Timecourses for additional mental health-related and fitness-related measures may be found in Figs. S18 , S19 , and S20 . Error ribbons in all panels denote the standard error of the mean. Horizontal lines below each panel’s timecourses denote intervals over which each weighted measure (color) differs from the unweighted baseline (via a paired sample two-sided t -test of the weighted mean values for each measure within a 30-day window around each timepoint; horizontal lines denote \(p < 0.05\) , corrected).
The above analyses indicate that recent differences in fitness-related activity are associated with differences in memory performance and mental health measures. Although the analyses treated these measures on average or in aggregate, many of the measures we collected are dynamic. For example, the amount or intensity of physical activity people engage in can vary over time, and so on. We wondered whether the dynamics of fitness-related measures might relate to memory performance and/or mental health measures. To this end, we carried out a series of reverse correlation analyses (see “ Reverse correlation analyses ”) to examine whether participants with different cognitive or mental health profiles also tended to display differences in fitness-related measures over time. In particular, we examined fitness data collected from participants’ Fitbit devices over the year prior to their test day in our study. Several example findings are summarized in Fig. 6 . We found that participants who performed well on the immediate and delayed free recall memory tests and on the naturalistic recall tests tended to be more active than participants who performed poorly on those tests (Fig. 6 a,b; Fig. S15 ). Conversely, participants who performed well on the immediate and delayed foreign language flashcards tasks tended to be less active. These differences were present even a full year before the testing day. We also found substantial variability across people with different (self-reported) mental health profiles (Fig. 6 ; Fig. S18 ). Due to small sample sizes of individuals exhibiting several mental health dimensions, it is difficult to distinguish generalizable trends from individual differences that one or two individuals happened to exhibit. However, several large-sample-size trends emerged. For example, participants who reported higher levels of stress also tended to be slightly more physically active than participants who reported lower stress levels. We found analogous differences in other activity-related measures (Figs. S15 and S18 ), cardiovascular measures (Figs. S16 and S19 ), and sleep-related measures (Figs. S17 and S20 ). Taken together, the analyses suggest that cognitive and mental health differences are also associated with differences in the dynamics of physical health measures.
After collecting a year’s worth of fitness-tracking data from each of 113 participants, we ran each participant in a battery of memory tasks and had them fill out a series of demographic and mental health-related questions. We found that the associations between fitness-related activities, memory performance, and mental health are complex. For example, participants who tended to engage in a particular intensity of physical activity also tended to perform better on some memory tasks but worse on others. This suggests that engaging in one form or intensity of physical activity will not necessarily affect all aspects of cognitive or mental health equally (or in the same direction).
A number of prior studies have shown that engaging in exercise can improve cognitive and mental health 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 . The majority of these studies ask participants in an “exercise intervention” condition (where participants engage in a designated physical activity or training regimen) or a “control” condition (where participants do not engage in the designated activity or training) to perform cognitive tasks or undergo mental health screening. In other words, most primary studies treat “physical activity” as a binary variable that either is or is not present for each participant. Most prior studies also track or manipulate exercise over relatively short durations (typically on the order of days or weeks). Our current work indicates that the true relations between physical activity, cognitive performance, and mental health may be non-monotonic and heterogeneous across activities, tasks, and mental health measures. These relations can also unfold over much longer timescales than have been previously identified (on the order of months; Fig. 6 ). However, despite the complexities of the structures of these associations, we also found that they were often remarkably consistent across people. For example, as displayed in Fig. 5 and Fig. S14 , many of the associations between fitness, behavioral, and mental health measures were consistent across over 97.5% of 10,000 randomly chosen subsets of participants.
One important limitation of our study is that we cannot distinguish correlations between different measures from potential causal effects. For example, we cannot know (from our study) whether engaging in particular forms of physical activity causes changes in memory performance or mental health, or whether (alternatively) people who tend to engage in similar forms of physical activity also happen to exhibit similar memory and/or mental health profiles. In other words, an overlapping set of processes or person-specific attributes may lead someone to both form particular habits around physical activity and display high or low performance on a given memory test. We do not know whether memory performance or aspects of mental health might be manipulated or influenced by changing the patterns of physical activity someone engages in. For this reason, we have been careful to frame our findings as correlations and associations, rather than to imply knowledge about causal directions of our findings.
Although the present study cannot reveal causal effects, a large prior literature provides some insight into potential causal effects by examining the neural and cognitive effects of a variety of exercise interventions 38 , 39 , 40 , 41 , 42 , 43 , 44 . A limitation of that prior work is that most of these studies examine how relatively short-term changes in physical activity (e.g., on timescales of hours to days or, rarely, weeks to months) affect a cognitive performance on single task or aspect of mental health. The present study examines longer-term physical activity (over a full year), and relates long-term physical activity history to performance on a variety of tasks and to a variety of mental health dimensions.
To the extent that physical activity does provide a non-invasive means of manipulating cognitive performance and mental health, our work may have exciting implications for cognitive enhancement. For example, one might imagine building a recommendation system that suggests a particular physical activity regimen tailored to improving a specific aspect of an individual’s cognitive performance (e.g., the efficacy of a student’s study session for an upcoming exam) or mental health (e.g., reducing symptoms of anxiety before an important meeting). Just as strength training may be customized to target a specific muscle group, or to improve performance on a specific physical task, similar principles might also be applied to target specific improvements in cognitive fitness and mental health.

Data availibility
All analysis code and data used in the present manuscript may be found at https://github.com/ContextLab/brainfit-paper .
Rogers, M. A. & Evans, W. J. Changes in skeletal muscle with aging: Effects of exercise training. Exerc. Sport Sci. Rev. 21 , 65–102 (1993).
Article CAS PubMed Google Scholar
Lindh, M. Increase of muscle strength from isometric quadriceps exercises at different knee angles. Scand. J. Rehabil. Med. 11 (1), 33–36 (1979).
CAS PubMed Google Scholar
Crane, J. D., MacNeil, L. G. & Tarnopolsky, M. A. Long-term aerobic exercise is associated with greater muscle strength throughout the life span. J. Gerontol. Ser. A 68 (6), 631–638 (2013).
Article Google Scholar
Knuttgen, H. G. Strength training and aerobic exercise: Comparison and contrast. J. Strength Cond. Res. 21 (3), 973–978 (2007).
PubMed Google Scholar
Chilibeck, P. D., Sale, D. G. & Webber, C. E. Exercise and bone mineral density. Sports Med. 19 , 103–122 (2012).
Bassey, E. J. & Ramsdale, S. J. Increase in femoral bone density in young women following high-impact exercise. Osteoporos. Int. 4 , 72–75 (1994).
Layne, J. E. & Nelson, M. E. The effects of progressive resistance training on bone density: A review. Med. Sci. Sports Exerc. 31 (1), 25–30 (1999).
Maiorana, A. et al. Combined aerobic and resistance exercise training improves functional capacity and strength in CHF. J. Appl. Physiol. 88 , 1565–1570 (2000).
Pollock, M. L. et al. Resistance exercise in individuals with and without cardiovascular disease. Circulation 101 , 828–833 (2000).
Lazovic-Popovic, B. et al. Superior lung capacity in swimmers: Some questions, more answers!. Rev. Port. Pneumol. 22 (3), 151–156 (2016).
Roman, M. A., Rossiter, H. B. & Casaburi, R. Exercise, ageing and the lung. Eur. Respir. J. 48 , 1471–1486 (2016).
Article PubMed Google Scholar
Wilmore, J. H. & Knuttgen, H. G. Aerobic exercise and endurance. Physician Sports Med. 31 (5), 45–51 (2003).
Raglin, J. S. Exercise and mental health. Sports Med. 9 , 323–329 (1990).
Mikkelsen, K., Stojanovska, L., Polenakovic, M., Bosevski, M. & Apostolopoulos, V. Exercise and mental health. Maturitas 106 , 48–56 (2017).
Taylor, C. B., Sallis, J. F. & Needle, R. The relation of physical activity and exercise to mental health. Public Health Rep. 100 (2), 195–202 (1985).
CAS PubMed PubMed Central Google Scholar
Deslandes, A. et al. Exercise and mental health: Many reasons to move. Neuropsychobiology 59 , 191–198 (2009).
Callaghan, P. Exercise: A neglected intervention in mental health care?. Psychiatr. Ment. Health Nurs. 11 (4), 476–483 (2004).
Article CAS Google Scholar
Paluska, S. A. & Schwenk, T. L. Physical activity and mental health. Sports Med. 29 (3), 167–180 (2000).
Basso, J. C. & Suzuki, W. A. The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: A review. Brain Plast. 2 (2), 127–152 (2017).
Article PubMed PubMed Central Google Scholar
Morres, I. D. et al. Aerobic exercise for adult patients with major depressive disorder in mental health services: A systematic review and meta-analysis. Depress. Anxiety 36 (1), 39–53 (2018).
Gordon, B. R., McDowell, C. P., Lyons, M. & Herring, M. P. The effects of resistance exercise training on anxiety: A meta-analysis and meta-regression analysis of randomized controlled trials. Sports Med. 47 (12), 2521–2532 (2017).
Morres, I. D. et al. Exercise for perinatal depressive symptoms: A systematic review and meta-analysis of randomized controlled trials in perinatal health services. J. Affect. Disord. 298 , 26–42 (2022).
Herring, M. P., O’Connor, P. J. & Dishman, R. K. The effects of exercise training on anxiety symptoms among patients: A systematic review. Arch. Intern. Med. 170 (4), 321–331 (2010).
Chang, Y. K., Labban, J. D., Gapin, J. I. & Etnier, J. L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 1453 , 87–101 (2012).
Brisswalter, J., Collardeau, M. & René, A. Effects of acute physical exercise characteristics on cognitive performance. Sports Med. 32 , 555–566 (2002).
Etnier, J. L., Nowell, P. M., Landers, D. M. & Sibley, B. A. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res. Brain Res. Rev. 52 (1), 119–130 (2006).
Morton, J. P., Kayani, A. C., McArdle, A. & Drust, B. The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med. 39 , 643–662 (2009).
Schiaffino, S., Dyar, K. A., Ciciliot, S., Blaauw, B. & Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 280 (17), 4294–4314 (2013).
Shoemaker, J. K., Halliwill, J. R., Hughson, R. L. & Joyner, M. J. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. Vasc. Physiol. 273 (5), 2388–2395 (1997).
Google Scholar
Palacios-Filardo, J. et al. Acetylcholine prioritises direct synaptic inputs from entorhinal cortex to CA1 by differential modulation of feedforward inhibitory circuits. Nat. Commun. 12 , 5475. https://doi.org/10.1038/s41467-021-25280-5 (2021).
Article ADS CAS PubMed PubMed Central Google Scholar
Gureckis, T. M. et al. psiTurk: An open-source framework for conducting replicable behavioral experiments online. Behav. Res. Methods 48 (3), 829–842 (2016).
Ziman, K., Heusser, A. C., Fitzpatrick, P. C., Field, C. E. & Manning, J. R. Is automatic speech-to-text transcription ready for use in psychological experiments?. Behav. Res. Methods 50 , 2597–2605 (2018).
Murdock, B. B. The serial position effect of free recall. J. Exp. Psychol. Gen. 64 , 482–488 (1962).
Kahana, M. J. Foundations of Human Memory (Oxford University Press, 2012).
Kahana, M. J. Associative retrieval processes in free recall. Mem. Cogn. 24 , 103–109 (1996).
Heusser, A. C., Fitzpatrick, P. C. & Manning, J. R. Geometric models reveal behavioral and neural signatures of transforming naturalistic experiences into episodic memories. Nat. Hum. Behav. 5 , 905–919 (2021).
Chen, J. et al. Shared memories reveal shared structure in neural activity across individuals. Nat. Neurosci. 20 (1), 115 (2017).
Chang, Y.-K. et al. Dose-response relation between exercise duration and cognition. Med. Sci. Sports Exerc. 47 , 159–165 (2015).
Vidoni, E. D. et al. Dose-response of aerobic exercise on cognition: A community-based, pilot randomized controlled trial. PLoS One 10 (7), 1–13 (2015).
Kamijo, K., Nishihira, Y., Higashiura, T. & Kuroiwa, K. The interactive effect of exercise intensity and task difficulty on human cognitive processing. Int. J. Psychophysiol. 65 (2), 114–121 (2007).
Imboden, C., Gerber, M., Beck, J., Eckert, A., Pühse, U., Holsboer-Trachsler, E. & Hatzinger, M. Effects of aerobic exercise as add-on treatment for inpatients with moderate to severe depression on depression severity, sleep, cognition, psychological well-being, and biomarkers: Study protocol, description of study population, and manipulation check. Front. Psychiatry 10 (262) (2019). https://doi.org/10.3389/fpsyt.2019.00262 .
Suwabe, K. et al. Acute moderate exercise improves mnemonic discrimination in young adults. Hippocampus 27 (3), 229–234 (2017).
Sinha, N., Berg, C. N., Yassa, M. A. & Gluck, M. A. Increased dynamic flexibility in the medial temporal lobe network following an exercise intervention mediates generalization of prior learning. Neurobiol. Learn. Mem. 177 , 107340 (2021).
Tomporowski, P. D. Effects of acute bouts of exercise on cognition. Acta Psychol. 112 (3), 297–324 (2003).
Download references
Acknowledgements
We acknowledge useful discussions with David Bucci, Emily Glasser, Andrew Heusser, Abigail Bartolome, Lorie Loeb, Lucy Owen, and Kirsten Ziman. Our work was supported in part by the Dartmouth Young Minds and Brains initiative, and by NSF grant number 2145172 to J.R.M. The content is solely the responsibility of the authors and does not necessarily represent the official views of our supporting organizations. This paper is dedicated to the memory of David Bucci, who helped to inspire the theoretical foundations of this work. Dave served as a mentor and colleague on the project prior to his passing.
Author information
Authors and affiliations.
Dartmouth College, Hanover, NH, USA
Jeremy R. Manning, Gina M. Notaro, Esme Chen & Paxton C. Fitzpatrick
You can also search for this author in PubMed Google Scholar
Contributions
Concept: J.R.M. and G.M.N. Experiment implementation and data collection: G.M.N. Analyses: J.R.M., G.M.N., E.C., and P.C.F. Writing: J.R.M. with input from all authors.
Corresponding author
Correspondence to Jeremy R. Manning .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Manning, J.R., Notaro, G.M., Chen, E. et al. Fitness tracking reveals task-specific associations between memory, mental health, and physical activity. Sci Rep 12 , 13822 (2022). https://doi.org/10.1038/s41598-022-17781-0
Download citation
Received : 02 March 2022
Accepted : 31 July 2022
Published : 15 August 2022
DOI : https://doi.org/10.1038/s41598-022-17781-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Predicting cognitive scores from wearable-based digital physiological features using machine learning: data from a clinical trial in mild cognitive impairment.
- Yuri G. Rykov
- Michael D. Patterson
- Nagaendran Kandiah
BMC Medicine (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Last updated 10th July 2024: Online ordering is currently unavailable due to technical issues. We apologise for any delays responding to customers while we resolve this. For further updates please visit our website https://www.cambridge.org/news-and-insights/technical-incident
We use cookies to distinguish you from other users and to provide you with a better experience on our websites. Close this message to accept cookies or find out how to manage your cookie settings .
Login Alert

- > Thinking through the Body
- > Muscle Memory and the Somaesthetic Pathologies of Everyday Life

Book contents
- Frontmatter
- Introduction
- Part I Somatic Being, Knowing, and Teaching
- 1 Thinking through the Body
- 2 The Body as Background
- 3 Self-Knowledge and Its Discontents
- 4 Muscle Memory and the Somaesthetic Pathologies of Everyday Life
- 5 Somaesthetics in the Philosophy Classroom
- Part II Somaesthetics, Aesthetics, and Culture
- Part III The Arts and the Art of Living
- Select Bibliography
4 - Muscle Memory and the Somaesthetic Pathologies of Everyday Life
Published online by Cambridge University Press: 05 November 2012
“Muscle memory” is a term commonly used in everyday discourse for the sort of embodied implicit memory that unconsciously helps us perform various motor tasks we have somehow learned through habituation, either through explicit, intentional training or simply as the result of informal, unintentional, or even unconscious learning from repeated prior experience. In scientific terminology, such memory is often designated as “procedural memory” or “motor memory” because it enables us to perform various motor procedures or skills in an automatic or spontaneous fashion, without conscious deliberation of how the procedure should be followed and without any explicit calculation of how one identifies and achieves the various steps involved in the procedure and how one proceeds from step to step. Paradigmatic of such muscle-memory motor skills of performance are walking, swimming, riding a bicycle, tying one's shoes, playing the piano, driving a car, or typing on a keyboard. To be precise, these motor skills should be described as sensorimotor, because they involve coordinating sensory perception with the movement of action. Moreover, because these skills apparently rely on schemata or patterns deeply embedded in an individual's central nervous system, the core engine of memory in so-called muscle memory is not simply the body's muscles but instead also involves the brain's neural networks.
The term “muscle memory” is nonetheless deeply entrenched, perhaps because it serves some key rhetorical functions. Muscle suggests body in contrast to mind, as muscular effort is frequently contrasted to mental effort or as muscle men are typically opposed to men of thought. Because of this common brain/brawn opposition, muscle memory conveys a sense of mindless memory. Such memory is mindless, however, only if we identify mind with mindfulness in the sense of explicit, critically focused consciousness or deliberate, reflective awareness. Procedural or performative tasks of implicit motor memory often require and exhibit significant mental skills and intelligence, as, for example, when a good pianist plays with spontaneity yet also with aesthetically sensitive mindfulness. In demonstrating that intelligent mind extends beyond clear consciousness, muscle memory also makes manifest the mind's embodied nature and the body's crucial role in memory and cognition.
Access options
Save book to kindle.
To save this book to your Kindle, first ensure [email protected] is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about saving to your Kindle .
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service .
- Muscle Memory and the Somaesthetic Pathologies of Everyday Life
- Richard Shusterman , Florida Atlantic University
- Book: Thinking through the Body
- Online publication: 05 November 2012
- Chapter DOI: https://doi.org/10.1017/CBO9781139094030.007
Save book to Dropbox
To save content items to your account, please confirm that you agree to abide by our usage policies. If this is the first time you use this feature, you will be asked to authorise Cambridge Core to connect with your account. Find out more about saving content to Dropbox .
Save book to Google Drive
To save content items to your account, please confirm that you agree to abide by our usage policies. If this is the first time you use this feature, you will be asked to authorise Cambridge Core to connect with your account. Find out more about saving content to Google Drive .
Stanford researchers observe memory formation in real time
By Alan Toth
Why is it that someone who hasn’t ridden a bicycle in decades can likely jump on and ride away without a wobble, but could probably not recall more than a name or two from their 3rd grade class?
This may be because physical skills — dubbed motor memories by neuroscientists — are encoded differently in our brains than our memories for names or facts.
Now, a new study by scientists with the Wu Tsai Neurosciences Institute is revealing exactly how motor memories are formed and why they are so persistent. It may even help illuminate the root causes of movement disorders like Parkinson’s disease.
“We think motor memory is unique,” said Jun Ding , an associate professor of neurosurgery and of neurology. “Some studies on Alzheimer’s disease included participants who were previously musicians and couldn’t remember their own families, but they could still play beautiful music. Clearly, there’s a huge difference in the way that motor memories are formed.”
Memories are thought to be encoded in the brain in the pattern of activity in networks of hundreds or thousands of neurons, sometimes distributed across distant brain regions. The concept of such a memory trace — sometimes called a memory engram — has been around for more than a century, but identifying exactly what an engram is and how it is encoded has proven extremely challenging. Previous studies have shown that some forms of learning activate specific neurons, which reactivate when the learned memory is recalled. However, whether memory engram neurons exist for motor skill learning remains unknown.
Ding and postdoctoral scholars Richard Roth and Fuu-Jiun Hwang wanted to know how these engram-like groups of cells get involved in learning and remembering a new motor skill.
“When you’re first learning to shoot a basketball, you use a very diverse set of neurons each time you throw, but as you get better, you use a more refined set that’s the same every time,” said Roth. “These refined neuron pathways were thought to be the basis of a memory engram, but we wanted to know exactly how these pathways emerge.”
In their new study, published July 8, 2022 in Neuron , the researchers trained mice to use their paws to reach food pellets through a small slot. Using genetic wizardry developed by the lab of Liqun Luo , a Wu Tsai Neurosciences Institute colleague in the Department of Biology, the researchers were able to identify specific neurons in the brain’s motor cortex — an area responsible for controlling movements — that were activated during the learning process. The researchers tagged these potential engram cells with a fluorescent marker so they could see if they also played a role in recalling the memory later on.
When the researchers tested the animals’ memory of this new skill weeks later, they found that those mice that still remembered the skill showed increased activity in the same neurons that were first identified during the learning period, showing that these neurons were responsible for encoding the skill: the researchers had observed the formation of memory engrams.
But how do these particular groups of neurons take on responsibility for learning a new task in the first place? And how do they actually improve the animal’s performance?
To answer these questions, the researchers zoomed in closer. Using two-photon microscopy to observe these living circuits in action, they observed the so-called “engram neurons” reprogram themselves as the mice learned. Motor cortex engram cells took on new synaptic inputs — potentially reflecting information about the reaching movement — and themselves formed powerful new output connections in a distant brain region called the dorsolateral striatum — a key waystation through which the engram neurons can exert refined control over the animal’s movements. It was the first time anyone had observed the creation of new synaptic pathways on the same neuron population — both at the input and the output levels — in these two brain regions.
The ability to trace new memories forming in the mouse brain allowed the research team to weigh in on a long-standing debate about how skills are stored in the brain: are they controlled from one central memory trace, or engram, or is the memory redundantly stored across many different brain areas? Though this study cannot discount the idea of centralized memory, it does lend credibility to the opposing theory. Another fascinating question is whether the activation of these engram neurons is required for the performance of already learned motor tasks. The researchers speculated that by suppressing the activity of neurons that had been identified as part of the motor cortex memory engram, the mice probably still would be able to perform the task.
“Think of memory like a highway. If 101 and 280 are both closed, you could still get to Stanford from San Francisco, it would just take a lot longer,” said Ding.
These findings suggest that, in addition to being dispersed, motor memories are highly redundant. The researchers say that as we repeat learned skills, we are continually reinforcing the motor engrams by building new connections — refining the skill. It’s what is meant by the term muscle memory — a refined, highly redundant network of motor engrams used so frequently that the associated skill seems automatic.

Ding believes that this constant repetition is one reason for the persistence of motor memory, but it’s not the only reason. Memory persistence may also be affected by a skill being associated with a reward, perhaps through the neurotransmitter dopamine. Though the research team did not directly address it in this study, Ding’s previous work in Parkinson’s disease suggests the connection.
“Current thinking is that Parkinson’s disease is the result of these motor engrams being blocked, but what if they’re actually being lost and people are forgetting these skills?” said Ding. “Remember that even walking is a motor skill that we all learned once, and it can potentially be forgotten.”
It’s a question that the researchers hope to answer in a follow-up study, because it may be the key to developing effective treatments for motor disorders. If Parkinson’s disease is the result of blocked motor memories, then patients should be able to improve their movement abilities by practicing and reinforcing these motor skills. On the other hand, if Parkinson’s destroys motor engrams and inhibits the creation of new ones — by targeting motor engram neurons and their synaptic connection observed in the team’s new study — then a completely different approach must be taken to deliver effective treatments.
“Our next goal is to understand what’s happening in movement disorders like Parkinson’s,” Ding said. “Obviously, we’re still a long way from a cure, but understanding how motor skills form is critical if we want to understand why they’re disrupted by disease.”
The research was published July 8 in Neuron: https://doi.org/10.1016/j.neuron.2022.06.006
Study authors were Fuu-Jiun Hwang, Richard H. Roth, Yu-Wei Wu, Yue Sun, Destany K. Kwon, Yu Liu, and Jun B. Ding.
The research was supported by the National Institutes of Health (NIH) and National Institute for Neurological Disease and Stroke (NINDS); the Klingenstein Foundation's Aligning Science Across Parkinson’s initiative; and GG gift fund, the Stanford School of Medicine Dean’s Postdoctoral Fellowship; and Parkinson’s Foundation Postdoctoral Fellowship.
Related stories
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Hum Neurosci
- PMC10410470
Cognitive neuroscience perspective on memory: overview and summary
Sruthi sridhar.
1 Department of Psychology, Mount Allison University, Sackville, NB, Canada
Abdulrahman Khamaj
2 Department of Industrial Engineering, College of Engineering, Jazan University, Jazan, Saudi Arabia
Manish Kumar Asthana
3 Department of Humanities and Social Sciences, Indian Institute of Technology Roorkee, Roorkee, India
4 Department of Design, Indian Institute of Technology Roorkee, Roorkee, India
Associated Data
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
This paper explores memory from a cognitive neuroscience perspective and examines associated neural mechanisms. It examines the different types of memory: working, declarative, and non-declarative, and the brain regions involved in each type. The paper highlights the role of different brain regions, such as the prefrontal cortex in working memory and the hippocampus in declarative memory. The paper also examines the mechanisms that underlie the formation and consolidation of memory, including the importance of sleep in the consolidation of memory and the role of the hippocampus in linking new memories to existing cognitive schemata. The paper highlights two types of memory consolidation processes: cellular consolidation and system consolidation. Cellular consolidation is the process of stabilizing information by strengthening synaptic connections. System consolidation models suggest that memories are initially stored in the hippocampus and are gradually consolidated into the neocortex over time. The consolidation process involves a hippocampal-neocortical binding process incorporating newly acquired information into existing cognitive schemata. The paper highlights the role of the medial temporal lobe and its involvement in autobiographical memory. Further, the paper discusses the relationship between episodic and semantic memory and the role of the hippocampus. Finally, the paper underscores the need for further research into the neurobiological mechanisms underlying non-declarative memory, particularly conditioning. Overall, the paper provides a comprehensive overview from a cognitive neuroscience perspective of the different processes involved in memory consolidation of different types of memory.
Introduction
Memory is an essential cognitive function that permits individuals to acquire, retain, and recover data that defines a person’s identity ( Zlotnik and Vansintjan, 2019 ). Memory is a multifaceted cognitive process that involves different stages: encoding, consolidation, recovery, and reconsolidation. Encoding involves acquiring and processing information that is transformed into a neuronal representation suitable for storage ( Liu et al., 2021 ; Panzeri et al., 2023 ). The information can be acquired through various channels, such as visual, auditory, olfactory, or tactile inputs. The acquired sensory stimuli are converted into a format the brain can process and retain. Different factors such as attention, emotional significance, and repetition can influence the encoding process and determine the strength and durability of the resulting memory ( Squire et al., 2004 ; Lee et al., 2016 ; Serences, 2016 ).
Consolidation includes the stabilization and integration of memory into long-term storage to increase resistance to interference and decay ( Goedert and Willingham, 2002 ). This process creates enduring structural modification in the brain and thereby has consequential effects on the function by reorganizing and strengthening neural connections. Diverse sources like sleep and stress and the release of neurotransmitters can influence memory consolidation. Many researchers have noted the importance of sleep due to its critical role in enabling a smooth transition of information from transient repositories into more stable engrams (memory traces) ( McGaugh, 2000 ; Clawson et al., 2021 ; Rakowska et al., 2022 ).
Retrieval involves accessing, selecting, and reactivating or reconstructing the stored memory to allow conscious access to previously encoded information ( Dudai, 2002 ). Retrieving memories depends on activating relevant neural pathways while reconstructing encoded information. Factors like contextual or retrieval cues and familiarity with the material can affect this process. Forgetting becomes a possibility if there are inadequate triggers for associated memory traces to activate upon recall. Luckily, mnemonic strategies and retrieval practice offer effective tools to enhance recovery rates and benefit overall memory performance ( Roediger and Butler, 2011 ).
Previous research implied that once a memory has been consolidated, it becomes permanent ( McGaugh, 2000 ; Robins, 2020 ). However, recent studies have found an additional phase called “reconsolidation,” during which stored memories, when reactivated, enter a fragile or liable state and become susceptible to modification or update ( Schiller et al., 2009 ; Asthana et al., 2015 ). The process highlights the notion that memory is not static but a dynamic system influenced by subsequent encounters. The concept of reconsolidation has much significance in memory modification therapies and interventions, as it offers a promising opportunity to target maladaptive or traumatic memories for modification specifically. However, more thorough investigations are needed to gain insight into the mechanisms and concrete implications of employing memory reconsolidation within therapeutic settings ( Bellfy and Kwapis, 2020 ).
The concept of memory is not reducible to a single unitary phenomenon; instead, evidence suggests that it can be subdivided into several distinct but interrelated constituent processes and systems ( Richter-Levin and Akirav, 2003 ). There are three major types of human memory: working memory, declarative memory (explicit), and non-declarative memory (implicit). All these types of memories involve different neural systems in the brain. Working memory is a unique transient active store capable of manipulating information essential for many complex cognitive operations, including language processing, reasoning, and judgment ( Atkinson and Shiffrin, 1968 ; Baddeley and Logie, 1999 ; Funahashi, 2017 ; Quentin et al., 2019 ). Previous models suggest the existence of three components that make up the working memory ( Baddeley and Hitch, 1974 ; Baddeley, 1986 ). One master component, the central executive, controls the two dependent components, the phonological loop (speech perception and language comprehension) and the visuospatial sketchpad (visual images and spatial impressions processing). Some models mention a third component known as the episodic buffer. It is theorized that the episodic buffer serves as an intermediary between perception, long-term memory, and two components of working memory (the phonological loop and visuospatial sketchpad) by storing integrated episodes or chunks from both sources ( Baddeley, 2000 ). Declarative memory (explicit memory) can be recalled consciously, including facts and events that took place in one’s life or information learned from books. It encompasses memories of both autobiographical experiences and memories associated with general knowledge. It is usually associated with the hippocampus–medial temporal lobe system ( Thompson and Kim, 1996 ; Ober, 2014 ). Non-declarative memory (implicit memory) refers to unconscious forms of learning such as skills, habits, and priming effects; this type of implicit learning does not involve conscious recollection but can include motor skill tasks that often require no thought prior to execution nor later recall upon completion. This type of memory usually involves the amygdala and other systems ( Thompson and Kim, 1996 ; Ober, 2014 ).
Working memory
Working memory is primarily associated with the prefrontal and posterior parietal cortex ( Sarnthein et al., 1998 ; Todd and Marois, 2005 ). Working memory is not localized to a single brain region, and research suggests that it is an emergent property arising from functional interactions between the prefrontal cortex (PFC) and the rest of the brain ( D’Esposito, 2007 ). Neuroimaging studies have explored the neural basis for the three components proposed by Baddeley and Hitch (1974) , the Central executive, the phonological loop, and the visuospatial sketch pad; there is evidence for the existence of a fourth component called the episodic buffer ( Baddeley, 2000 ).
The central executive plays a significant role in working memory by acting as the control center ( Shallice, 2002 ). It facilitates critical functions like attention allocation and coordination between the phonological loop and the visuospatial sketchpad ( Yu et al., 2023 ). Recent findings have illuminated the dual-functional network regulation, the cingulo-opercular network (CON) and the frontoparietal network (FPN), that underpins the central executive system ( Yu et al., 2023 ). The CON comprises the dorsal anterior cingulate cortex (dACC) and anterior insula (AI). In contrast, the FPN encompasses various regions, such as the dorsolateral prefrontal cortex (DLPFC) and frontal eye field (FEF), along with the intraparietal sulcus (IPS) ( Yu et al., 2023 ). Neuroimaging research has found evidence that elucidates the neural underpinnings of the executive attention control system to the dorsolateral prefrontal cortex (DLPFC) and the anterior cingulate cortex (ACC) ( Jung et al., 2022 ). The activation patterns indicate that the CON may have a broader top-down control function across the working memory process. At the same time, the FPN could be more heavily implicated in momentary control or processing at the trial level ( Yu et al., 2023 ). Evidence suggests that the central executive interacts with the phonological loop and visuospatial sketchpad to support working memory processes ( Baddeley, 2003 ; Buchsbaum, 2010 ; Menon and D’Esposito, 2021 ). The function, localization, and neural basis of this interaction are thought to involve the activation of specific brain regions associated with each component of working memory, as discussed in detail below.
The phonological loop is divided into two components: a storage system that maintains information (a few seconds) and a component involving subvocal rehearsal—which maintains and refreshes information in the working memory. Neuroanatomically, the phonological loop is represented in the Brodmann area (BA) 40 in the parietal cortex and the rehearsal components in BA 44 and 6, both situated in the frontal cortex ( Osaka et al., 2007 ). The left inferior frontal gyrus (Broca’s area) and the left posterior superior temporal gyrus (Wernicke’s area) has been proposed to play a critical role in supporting phonological and verbal working memory tasks, specifically the subvocal rehearsal system of the articulatory loop ( Paulesu et al., 1993 ; Buchsbaum et al., 2001 ; Perrachione et al., 2017 ). The phonological store in verbal short-term memory has been localized at the left supramarginal gyrus ( Graves et al., 2008 ; Perrachione et al., 2017 ).
Studies utilizing neuroimaging techniques have consistently yielded results indicating notable activation in these brain regions during phonological activities like recalling non-words and maintaining verbal information in memory ( Awh et al., 1996 ; Graves et al., 2008 ). During tasks that require phonological rehearsal, there was an increase in activation in the left inferior frontal gyrus ( Paulesu et al., 1993 ). Researchers have noted an increase in activity within the superior temporal gyrus-which plays a significant role in auditory processing-in individuals performing tasks that necessitate verbal information maintenance and manipulation ( Smith et al., 1998 ; Chein et al., 2003 ).
Additionally, lesion studies have provided further confirmation regarding the importance of these regions. These investigations have revealed that impairment in performing phonological working memory tasks can transpire following damage inflicted upon the left hemisphere, particularly on perisylvian language areas ( Koenigs et al., 2011 ). It is common for individuals with lesions affecting regions associated with the phonological loop, such as the left inferior frontal gyrus and superior temporal gyrus, to have difficulty performing verbal working memory tasks. Clinical cases involving patients diagnosed with aphasia and specific language impairments have highlighted challenges related to retaining and manipulating auditory information. For example, those who sustain damage specifically within their left inferior frontal gyrus often struggle with tasks involving phonological rehearsal and verbal working memory activities, and therefore, they tend to perform poorly in tasks that require manipulation or repetition of verbal stimuli ( Saffran, 1997 ; Caplan and Waters, 2005 ).
The visuospatial sketchpad is engaged in the temporary retention and manipulation of visuospatial facts, including mental pictures, spatial associations, and object placements ( Miyake et al., 2001 ). The visuospatial sketchpad is localized to the right hemisphere, including the occipital lobe, parietal and frontal areas ( Osaka et al., 2007 ). Ren et al. (2019) identified the localization of the visuospatial sketchpad, and these areas were the right infero-lateral prefrontal cortex, lateral pre-motor cortices, right inferior parietal cortex, and the dorsolateral occipital cortices ( Burbaud et al., 1999 ; Salvato et al., 2021 ). Moreover, the posterior parietal cortex and the intraparietal sulcus have been implicated in spatial working memory ( Xu and Chun, 2006 ). Additionally, some evidence is available for an increase in brain regions associated with the visuospatial sketchpad during tasks involving mental imagery and spatial processing. Neuroimaging studies have revealed increased neural activation in some regions of the parietal cortex, mainly the superior and posterior parietal cortex, while performing mental rotation tasks ( Cohen et al., 1996 ; Kosslyn et al., 1997 ). However, further research is needed to better understand the visuospatial working memory and its integration with other cognitive processes ( Baddeley, 2003 ). Lesions to the regions involving the visuospatial sketchpad can have detrimental effects on visuospatial working memory tasks. Individuals with lesions to the posterior parietal cortex may exhibit deficits in mental rotation tasks and may be unable to mentally manipulate the visuospatial representation ( Buiatti et al., 2011 ). Moreover, studies concerning lesions have shown that damage to the parietal cortex can result in short-term deficits in visuospatial memory ( Shafritz et al., 2002 ). Damage to the occipital cortex can lead to performance impairments in tasks that require the generation and manipulation of mental visual images ( Moro et al., 2008 ).
The fourth component of the working memory, termed episodic buffer, was proposed by Baddeley (2000) . The episodic buffer is a multidimensional but essentially passive store that can hold a limited number of chunks, store bound features, and make them available to conscious awareness ( Baddeley et al., 2010 ; Hitch et al., 2019 ). Although research has suggested that episodic buffer is localized to the hippocampus ( Berlingeri et al., 2008 ) or the inferior lateral parietal cortex, it is thought to be not dependent on a single anatomical structure but instead can be influenced by the subsystems of working memory, long term memory, and even through perception ( Vilberg and Rugg, 2008 ; Baddeley et al., 2010 ). The episodic buffer provides a crucial link between the attentional central executive and the multidimensional information necessary for the operation of working memory ( Baddeley et al., 2011 ; Gelastopoulos et al., 2019 ).
The interdependence of the working memory modules, namely the phonological loop and visuospatial sketchpad, co-relates with other cognitive processes, for instance, spatial cognition and attention allocation ( Repovs and Baddeley, 2006 ). It has been found that the prefrontal cortex (PFC) and posterior parietal cortex (PPC) have a crucial role in several aspects of spatial cognition, such as the maintenance of spatially oriented attention and motor intentions ( Jerde and Curtis, 2013 ). The study by Sellers et al. (2016) and the review by Ikkai and Curtis (2011) posits that other brain areas could use the activity in PFC and PPC as a guide and manifest outputs to guide attention allocation, spatial memory, and motor planning. Moreover, research indicates that verbal information elicits an activation response in the left ventrolateral prefrontal cortex (VLPFC) when retained in the phonological loop, while visuospatial information is represented by a corresponding level of activity within the right homolog region ( Narayanan et al., 2005 ; Wolf et al., 2006 ; Emch et al., 2019 ). Specifically, the study by Yang et al. (2022) investigated the roles of two regions in the brain, the right inferior frontal gyrus (rIFG) and the right supra-marginal gyrus (rSMG), as they relate to spatial congruency in visual working memory tasks. A change detection task with online repetitive transcranial magnetic stimulation applied concurrently at both locations during high visual WM load conditions determined that rIFG is involved in actively repositioning the location of objects. At the same time, rSMG is engaged in passive perception of the stability of the location of objects.
Recent academic studies have found evidence to support the development of a new working memory model known as the state-based model ( D’Esposito and Postle, 2015 ). This theoretical model proposes that the allocation of attention toward internal representations permits short-term retention within working memory ( Ghaleh et al., 2019 ). The state-based model consists of two main categories: activated LTM models and sensorimotor recruitment models; the former largely focuses upon symbolic stimuli categorized under semantic aspects, while the latter has typically been applied to more perceptual tasks in experiments. This framework posits that prioritization through regulating cognitive processes provides insight into various characteristics across different activity types, including capacity limitations, proactive interference, etcetera ( D’Esposito and Postle, 2015 ). For example, the paper by Ghaleh et al. (2019) provides evidence for two separate mechanisms involved in maintenance of auditory information in verbal working memory: an articulatory rehearsal mechanism that relies more heavily on left sensorimotor areas and a non-articulatory maintenance mechanism that critically relies on left superior temporal gyrus (STG). These findings support the state-based model’s proposal that attentional allocation is necessary for short-term retention in working memory.
State-based models were found to be consistent with the suggested storage mechanism as they do not require representation transfer from one dedicated buffer type; research has demonstrated that any population of neurons and synapses may serve as such buffers ( Maass and Markram, 2002 ; Postle, 2006 ; Avraham et al., 2017 ). The review by D’Esposito and Postle (2015) examined the evidence to determine whether a persistent neural activity, synaptic mechanisms, or a combination thereof support representations maintained during working memory. Numerous neural mechanisms have been hypothesized to support the short-term retention of information in working memory and likely operate in parallel ( Sreenivasan et al., 2014 ; Kamiński and Rutishauser, 2019 ).
Persistent neural activity is the neural mechanism by which information is temporarily maintained ( Ikkai and Curtis, 2011 ; Panzeri et al., 2023 ). Recent review by Curtis and Sprague (2021) has focused on the notion that persistent neural activity is a fundamental mechanism for memory storage and have provided two main arcs of explanation. The first arc, mainly underpinned by empirical evidence from prefrontal cortex (PFC) neurophysiology experiments and computational models, posits that PFC neurons exhibit sustained firing during working memory tasks, enabling them to store representations in their active state ( Thuault et al., 2013 ). Intrinsic persistent firing in layer V neurons in the medial PFC has been shown to be regulated by HCN1 channels, which contribute to the executive function of the PFC during working memory episodes ( Thuault et al., 2013 ). Additionally, research has also found that persistent neural firing could possibly interact with theta periodic activity to sustain each other in the medial temporal, prefrontal, and parietal regions ( Düzel et al., 2010 ; Boran et al., 2019 ). The second arc involves advanced neuroimaging approaches which have, more recently, enabled researchers to decode content stored within working memories across distributed regions of the brain, including parts of the early visual cortex–thus extending this framework beyond just isolated cortical areas such as the PFC. There is evidence that suggests simple, stable, persistent activity among neurons in stimulus-selective populations may be a crucial mechanism for sustaining WM representations ( Mackey et al., 2016 ; Kamiński et al., 2017 ; Curtis and Sprague, 2021 ).
Badre (2008) discussed the functional organization of the PFC. The paper hypothesized that the rostro-caudal gradient of a function in PFC supported a control hierarchy, whereas posterior to anterior PFC mediated progressively abstract, higher-order controls ( Badre, 2008 ). However, this outlook proposed by Badre (2008) became outdated; the paper by Badre and Nee (2018) presented an updated look at the literature on hierarchical control. This paper supports neither a unitary model of lateral frontal function nor a unidimensional abstraction gradient. Instead, separate frontal networks interact via local and global hierarchical structures to support diverse task demands. This updated perspective is supported by recent studies on the hierarchical organization of representations within the lateral prefrontal cortex (LPFC) and the progressively rostral areas of the LPFC that process/represent increasingly abstract information, facilitating efficient and flexible cognition ( Thomas Yeo et al., 2011 ; Nee and D’Esposito, 2016 ). This structure allows the brain to access increasingly abstract action representations as required ( Nee and D’Esposito, 2016 ). It is supported by fMRI studies showing an anterior-to-posterior activation movement when tasks become more complex. Anatomical connectivity between areas also supports this theory, such as Area 10, which has projections back down to Area 6 but not vice versa.
Finally, studies confirm that different regions serve different roles along a hierarchy leading toward goal-directed behavior ( Badre and Nee, 2018 ). The paper by Postle (2015) exhibits evidence of activity in the prefrontal cortex that reflects the maintenance of high-level representations, which act as top-down signals, and steer the circulation of neural pathways across brain networks. The PFC is a source of top-down signals that influence processing in the posterior and subcortical regions ( Braver et al., 2008 ; Friedman and Robbins, 2022 ). These signals either enhance task-relevant information or suppress irrelevant stimuli, allowing for efficient yet effective search ( D’Esposito, 2007 ; D’Esposito and Postle, 2015 ; Kerzel and Burra, 2020 ). The study by Ratcliffe et al. (2022) provides evidence of the dynamic interplay between executive control mechanisms in the frontal cortex and stimulus representations held in posterior regions for working memory tasks. Moreover, the review by Herry and Johansen (2014) discusses the neural mechanisms behind actively maintaining task-relevant information in order for a person to carry out tasks and goals effectively. This review of data and research suggests that working memory is a multi-component system allowing for both the storage and processing of temporarily active representations. Neural activity throughout the brain can be differentially enhanced or suppressed based on context through top-down signals emanating from integrative areas such as PFC, parietal cortex, or hippocampus to actively maintain task-relevant information when it is not present in the environment ( Herry and Johansen, 2014 ; Kerzel and Burra, 2020 ).
In addition, Yu et al. (2022) examined how brain regions from the ventral stream pathway to the prefrontal cortex were activated during working memory (WM) gate opening and closing. They defined gate opening as the switch from maintenance to updating and gate closing as the switch from updating to maintenance. The data suggested that cognitive branching increases during the WM gating process, thus correlating the gating process and an information approach to the PFC function. The temporal cortices, lingual gyrus (BA19), superior frontal gyri including frontopolar cortices, and middle and inferior parietal regions are involved in processes of estimating whether a response option available will be helpful for each case. During gate closing, on the other hand, medial and superior frontal regions, which have been associated with conflict monitoring, come into play, as well as orbitofrontal and dorsolateral prefrontal processing at later times when decreasing activity resembling stopping or downregulating cognitive branching has occurred, confirming earlier theories about these areas being essential for estimation of usefulness already stored within long-term memories ( Yu et al., 2022 ).
Declarative and non-declarative memory
The distinctions between declarative and non-declarative memory are often based on the anatomical features of medial temporal lobe regions, specifically those involving the hippocampus ( Squire and Zola, 1996 ; Squire and Wixted, 2011 ). In the investigation of systems implicated in the process of learning and memory formation, it has been posited that the participation of the hippocampus is essential for the acquisition of declarative memories ( Eichenbaum and Cohen, 2014 ). In contrast, a comparatively reduced level of hippocampal involvement may suffice for non-declarative memories ( Squire and Zola, 1996 ; Williams, 2020 ).
Declarative memory (explicit) pertains to knowledge about facts and events. This type of information can be consciously retrieved with effort or spontaneously recollected without conscious intention ( Dew and Cabeza, 2011 ). There are two types of declarative memory: Episodic and Semantic. Episodic memory is associated with the recollection of personal experiences. It involves detailed information about events that happened in one’s life. Semantic memory refers to knowledge stored in the brain as facts, concepts, ideas, and objects; this includes language-related information like meanings of words and mathematical symbol values along with general world knowledge (e.g., capitals of countries) ( Binder and Desai, 2011 ). The difference between episodic and semantic memory is that when one retrieves episodic memory, the experience is known as “remembering”; when one retrieves information from semantic memory, the experience is known as “knowing” ( Tulving, 1985 ; Dew and Cabeza, 2011 ). The hippocampus, medial temporal lobe, and the areas in the diencephalon are implicated in declarative memory ( Richter-Levin and Akirav, 2003 ; Derner et al., 2020 ). The ventral parietal cortex (VPC) is involved in declarative memory processes, specifically episodic memory retrieval ( Henson et al., 1999 ; Davis et al., 2018 ). The evidence suggests that VPC and hippocampus is involved in the retrieval of contextual details, such as the location and timing of the event, and the information is critical for the formation of episodic memory ( Daselaar, 2009 ; Hutchinson et al., 2009 ; Wiltgen et al., 2010 ). The prefrontal cortex (PFC) is involved in the encoding (medial PFC) and retrieval (lateral PFC) of declarative memories, specifically in the integration of information across different sensory modalities ( Blumenfeld and Ranganath, 2007 ; Li et al., 2010 ). Research also suggests that the amygdala may modulate other brain regions involved with memory processing, thus, contributing to an enhanced recall of negative or positive experiences ( Hamann, 2001 ; Ritchey et al., 2008 ; Sendi et al., 2020 ). Maintenance of the integrity of hippocampal circuitry is essential for ensuring that episodic memory, along with spatial and temporal context information, can be retained in short-term or long-term working memory beyond 15 min ( Ito et al., 2003 ; Rasch and Born, 2013 ). Moreover, studies have suggested that the amygdala plays a vital role in encoding and retrieving explicit memories, particularly those related to emotionally charged stimuli which are supported by evidence of correlations between hippocampal activity and amygdala modulation during memory formation ( Richter-Levin and Akirav, 2003 ; Qasim et al., 2023 ).
Current findings in neuroimaging studies assert that a vast array of interconnected brain regions support semantic memory ( Binder and Desai, 2011 ). This network merges information sourced from multiple senses alongside different cognitive faculties necessary for generating abstract supramodal views on various topics stored within our consciousness. Modality-specific sensory, motor, and emotional system within these brain regions serve specialized tasks like language comprehension, while larger areas of the brain, such as the inferior parietal lobe and most of the temporal lobe, participate in more generalized interpretation tasks ( Binder and Desai, 2011 ; Kuhnke et al., 2020 ). These regions lie at convergences of multiple perceptual processing streams, enabling increasingly abstract, supramodal representations of perceptual experience that support a variety of conceptual functions, including object recognition, social cognition, language, and the remarkable human capacity to remember the past and imagine the future ( Binder and Desai, 2011 ; Binney et al., 2016 ). The following section will discuss the processes underlying memory consolidation and storage within declarative memory.
Non-declarative (implicit) memories refer to unconscious learning through experience, such as habits and skills formed from practice rather than memorizing facts; these are typically acquired slowly and automatically in response to sensory input associated with reward structures or prior exposure within our daily lives ( Kesner, 2017 ). Non-declarative memory is a collection of different phenomena with different neural substrates rather than a single coherent system ( Camina and Güell, 2017 ). It operates by similar principles, depending on local changes to a circumscribed brain region, and the representation of these changes is unavailable to awareness ( Reber, 2008 ). Non-declarative memory encompasses a heterogenous collection of abilities, such as associative learning, skills, and habits (procedural memory), priming, and non-associative learning ( Squire and Zola, 1996 ; Camina and Güell, 2017 ). Studies have concluded that procedural memory for motor skills depends upon activity in diverse set areas such as the motor cortex, striatum, limbic system, and cerebellum; similarly, perceptual skill learning is thought to be associated with sensory cortical activation ( Karni et al., 1998 ; Mayes, 2002 ). Research suggests that mutual connections between brain regions that are active together recruit special cells called associative memory cells ( Wang et al., 2016 ; Wang and Cui, 2018 ). These cells help integrate, store, and remember related information. When activated, these cells trigger the recall of memories, leading to behaviors and emotional responses. This suggests that co-activated brain regions with these mutual connections are where associative memories are formed ( Wang et al., 2016 ; Wang and Cui, 2018 ). Additionally, observational data reveals that priming mechanisms within distinct networks, such as the “repetition suppression” effect observed in visual cortical areas associated with sensory processing and in the prefrontal cortex for semantic priming, are believed to be responsible for certain forms of conditioning and implicit knowledge transfer experiences exhibited by individuals throughout their daily lives ( Reber, 2008 ; Wig et al., 2009 ; Camina and Güell, 2017 ). However, further research is needed to better understand the mechanisms of consolidation in non-declarative memory ( Camina and Güell, 2017 ).
The process of transforming memory into stable, long-lasting from a temporary, labile memory is known as memory consolidation ( McGaugh, 2000 ). Memory formation is based on the change in synaptic connections of neurons representing the memory. Encoding causes synaptic Long-Term potentiation (LTP) or Long-Term depression (LTD) and induces two consolidation processes. The first is synaptic or cellular consolidation which involves remodeling synapses to produce enduring changes. Cellular consolidation is a short-term process that involves stabilizing the neural trace shortly after learning via structural brain changes in the hippocampus ( Lynch, 2004 ). The second is system consolidation, which builds on synaptic consolidation where reverberating activity leads to redistribution for long-term storage ( Mednick et al., 2011 ; Squire et al., 2015 ). System consolidation is a long-term process during which memories are gradually transferred to and integrated with cortical neurons, thus promoting their stability over time. In this way, memories are rendered less susceptible to forgetting. Hebb postulated that when two neurons are repeatedly activated simultaneously, they become more likely to exhibit a coordinated firing pattern of activity in the future ( Langille, 2019 ). This proposed enduring change in synchronized neuronal activation was consequently termed cellular consolidation ( Bermudez-Rattoni, 2010 ).
The following sections of this paper incorporate a more comprehensive investigation into various essential procedures connected with memory consolidation- namely: long-term potentiation (LTP), long-term depression (LTD), system consolidation, and cellular consolidation. Although these mechanisms have been presented briefly before this paragraph, the paper aims to offer greater insight into each process’s function within the individual capacity and their collective contribution toward memory consolidation.
Synaptic plasticity mechanisms implicated in memory stabilization
Long-Term Potentiation (LTP) and Long-Term Depression (LTP) are mechanisms that have been implicated in memory stabilization. LTP is an increase in synaptic strength, whereas LTD is a decrease in synaptic strength ( Ivanco, 2015 ; Abraham et al., 2019 ).
Long-Term Potentiation (LTP) is a phenomenon wherein synaptic strength increases persistently due to brief exposures to high-frequency stimulation ( Lynch, 2004 ). Studies of Long-Term Potentiation (LTP) have led to an understanding of the mechanisms behind synaptic strengthening phenomena and have provided a basis for explaining how and why strong connections between neurons form over time in response to stimuli.
The NMDA receptor-dependent LTP is the most commonly described LTP ( Bliss and Collingridge, 1993 ; Luscher and Malenka, 2012 ). In this type of LTP, when there is high-frequency stimulation, the presynaptic neuron releases glutamate, an excitatory neurotransmitter. Glutamate binds to the AMPA receptor on the postsynaptic neuron, which causes the neuron to fire while opening the NMDA receptor channel. The opening of an NMDA channel elicits a calcium ion influx into the postsynaptic neuron, thus initiating a series of phosphorylation events as part of the ensuing molecular cascade. Autonomously phosphorylated CaMKII and PKC, both actively functional through such a process, have been demonstrated to increase the conductance of pre-existing AMPA receptors in synaptic networks. Additionally, this has been shown to stimulate the introduction of additional AMPA receptors into synapses ( Malenka and Nicoll, 1999 ; Lynch, 2004 ; Luscher and Malenka, 2012 ; Bailey et al., 2015 ).
There are two phases of LTP: the early phase and the late phase. It has been established that the early phase LTP (E-LTP) does not require RNA or protein synthesis; therefore, its synaptic strength will dissipate in minutes if late LTP does not stabilize it. On the contrary, late-phase LTP (L-LTP) can sustain itself over a more extended period, from several hours to multiple days, with gene transcription and protein synthesis in the postsynaptic cell ( Frey and Morris, 1998 ; Orsini and Maren, 2012 ). The strength of presynaptic tetanic stimulation has been demonstrated to be a necessary condition for the activation of processes leading to late LTP ( Luscher and Malenka, 2012 ; Bailey et al., 2015 ). This finding is supported by research examining synaptic plasticity, notably Eric Kandel’s discovery that CREB–a transcription factor–among other cytoplasmic and nuclear molecules, are vital components in mediating molecular changes culminating in protein synthesis during this process ( Kaleem et al., 2011 ; Kandel et al., 2014 ). Further studies have shown how these shifts ultimately lead to AMPA receptor stabilization at post-synapses facilitating long-term potentiation within neurons ( Luscher and Malenka, 2012 ; Bailey et al., 2015 ).
The “synaptic tagging and capture hypothesis” explains how a weak event of tetanization at synapse A can transform to late-LTP if followed shortly by the strong tetanization of a different, nearby synapse on the same neuron ( Frey and Morris, 1998 ; Redondo and Morris, 2011 ; Okuda et al., 2020 ; Park et al., 2021 ). During this process, critical plasticity-related proteins (PRPs) are synthesized, which stabilize their own “tag” and that from the weaker synaptic activity ( Moncada et al., 2015 ). Recent evidence suggests that calcium-permeable AMPA receptors (CP-AMPARs) are involved in this form of heterosynaptic metaplasticity ( Park et al., 2018 ). The authors propose that the synaptic activation of CP-AMPARs triggers the synthesis of PRPs, which are then engaged by the weak induction protocol to facilitate LTP on the independent input. The paper also suggests that CP-AMPARs are required during the induction of LTP by the weak input for the full heterosynaptic metaplastic effect to be observed ( Park et al., 2021 ). Additionally, it has been further established that catecholamines such as dopamine plays an integral part in memory persistence by inducing PRP synthesis ( Redondo and Morris, 2011 ; Vishnoi et al., 2018 ). Studies have found that dopamine release in the hippocampus can enhance LTP and improve memory consolidation ( Lisman and Grace, 2005 ; Speranza et al., 2021 ).
Investigations into neuronal plasticity have indicated that synaptic strength alterations associated with certain forms of learning and memory may be analogous to those underlying Long-Term Potentiation (LTP). Research has corroborated this notion, demonstrating a correlation between these two phenomena ( Lynch, 2004 ). The three essential properties of Long-Term Potentiation (LTP) that have been identified are associativity, synapse specificity, and cooperativity ( Kandel and Mack, 2013 ). These characteristics provide empirical evidence for the potential role of LTP in memory formation processes. Specifically, associativity denotes the amplification of connections when weak stimulus input is paired with a powerful one; synapse specificity posits that this potentiating effect only manifests on synaptic locations exhibiting coincidental activity within postsynaptic neurons, while cooperativity suggests stimulated neuron needs to attain an adequate threshold of depolarization before LTP can be induced again ( Orsini and Maren, 2012 ).
There is support for the idea that memories are encoded by modification of synaptic strengths through cellular mechanisms such as LTP and LTD ( Nabavi et al., 2014 ). The paper by Nabavi et al. (2014) shows that fear conditioning, a type of associative memory, can be inactivated and reactivated by LTD and LTP, respectively. The findings of the paper support a causal link between these synaptic processes and memory. Moreover, the paper suggests that LTP is used to form neuronal assemblies that represent a memory, and LTD could be used to disassemble them and thereby inactivate a memory ( Nabavi et al., 2014 ). Hippocampal LTD has been found to play an essential function in regulating synaptic strength and forming memories, such as long-term spatial memory ( Ge et al., 2010 ). However, it is vital to bear in mind that studies carried out on LTP exceed those done on LTD; hence the literature on it needs to be more extensive ( Malenka and Bear, 2004 ; Nabavi et al., 2014 ).
Cellular consolidation and memory
For an event to be remembered, it must form physical connections between neurons in the brain, which creates a “memory trace.” This memory trace can then be stored as long-term memory ( Langille and Brown, 2018 ). The formation of a memory engram is an intricate process requiring neuronal depolarization and the influx of intracellular calcium ( Mank and Griesbeck, 2008 ; Josselyn et al., 2015 ; Xu et al., 2017 ). This initiation leads to a cascade involving protein transcription, structural and functional changes in neural networks, and stabilization during the quiescence period, followed by complete consolidation for its success. Interference from new learning events or disruption caused due to inhibition can abort this cycle leading to incomplete consolidation ( Josselyn et al., 2015 ).
Cyclic-AMP response element binding protein (CREB) has been identified as an essential transcription factor for memory formation ( Orsini and Maren, 2012 ). It regulates the expression of PRPs and enhances neuronal excitability and plasticity, resulting in changes to the structure of cells, including the growth of dendritic spines and new synaptic connections. Blockage or enhancement of CREB in certain areas can affect subsequent consolidation at a systems level–decreasing it prevents this from occurring, while aiding its presence allows even weak learning conditions to produce successful memory formation ( Orsini and Maren, 2012 ; Kandel et al., 2014 ).
Strengthening weakly encoded memories through the synaptic tagging and capture hypothesis may play an essential role in cellular consolidation. Retroactive memory enhancement has also been demonstrated in human studies, mainly when items are initially encoded with low strength but later paired with shock after consolidation ( Dunsmoor et al., 2015 ). The synaptic tagging and capture theory (STC) and its extension, the behavioral tagging hypothesis (BT), have both been used to explain synaptic specificity and the persistence of plasticity ( Moncada et al., 2015 ). STC proposed that electrophysiological activity can induce long-term changes in synapses, while BT postulates similar effects of behaviorally relevant neuronal events on learning and memory models. This hypothesis proposes that memory consolidation relies on combining two distinct processes: setting a “learning tag” and synthesizing plasticity-related proteins ( De novo protein synthesis, increased CREB levels, and substantial inputs to nearby synapses) at those tagged sites. BT explains how it is possible for event episodes with low-strength inputs or engagements can be converted into lasting memories ( Lynch, 2004 ; Moncada et al., 2015 ). Similarly, the emotional tagging hypothesis posits that the activation of the amygdala in emotionally arousing events helps to mark experiences as necessary, thus enhancing synaptic plasticity and facilitating transformation from transient into more permanent forms for encoding long-term memories ( Richter-Levin and Akirav, 2003 ; Zhu et al., 2022 ).
Cellular consolidation, the protein synthesis-dependent processes observed in rodents that may underlie memory formation and stabilization, has been challenging to characterize in humans due to the limited ability to study it directly ( Bermudez-Rattoni, 2010 ). Additionally, multi-trial learning protocols commonly used within human tests as opposed to single-trial experiments conducted with non-human subjects suggest there could be interference from subsequent information that impedes individual memories from being consolidated reliably. This raises important questions regarding how individuals can still form strong and long-lasting memories when exposed to frequent stimuli outside controlled laboratory conditions. Although this phenomenon remains undiscovered by science, it is of utmost significance for gaining a deeper understanding of our neural capacities ( Genzel and Wixted, 2017 ).
The establishment of distributed memory traces requires a narrow temporal window following the initial encoding process, during which cellular consolidation occurs ( Nader and Hardt, 2009 ). Once this period ends and consolidation has been completed, further protein synthesis inhibition or pharmacological disruption will be less effective at altering pre-existing memories and interfering with new learning due to the stabilization of the trace in its new neuronal network connections ( Nader and Hardt, 2009 ). Thus, systems consolidation appears critical for the long-term maintenance of memory within broader brain networks over extended periods after their formation ( Bermudez-Rattoni, 2010 ).
System consolidation and memory
Information is initially stored in both the hippocampus and neocortex ( Dudai et al., 2015 ). The hippocampus subsequently guides a gradual process of reorganization and stabilization whereby information present within the neocortex becomes autonomous from that in the hippocampal store. Scholars have termed this phenomenon “standard memory consolidation model” or “system consolidation” ( Squire et al., 2015 ).
The Standard Model suggests that information acquired during learning is simultaneously stored in both the hippocampus and multiple cortical modules. Subsequently, it posits that over a period of time which may range from weeks to months or longer, the hippocampal formation directs an integration process by which these various elements become enclosed into single unified structures within the cortex ( Gilboa and Moscovitch, 2021 ; Howard et al., 2022 ). These newly learned memories are then assimilated into existing networks without interference or compression when necessary ( Frankland and Bontempi, 2005 ). It is important to note that memory engrams already exist within cortical networks during encoding. They only need strengthening through links enabled by hippocampal assistance-overtime allowing remote memory storage without reliance on the latter structure. Data appears consistent across studies indicating that both AMPA-and NMDA receptor-dependent “tagging” processes occurring within the cortex are essential components of progressive rewiring, thus enabling longer-term retention ( Takeuchi et al., 2014 ; Takehara-Nishiuchi, 2020 ).
Recent studies have additionally demonstrated that the rate of system consolidation depends on an individual’s ability to relate new information to existing networks made up of connected neurons, popularly known as “schemas” ( Robin and Moscovitch, 2017 ). In situations where prior knowledge is present and cortical modules are already connected at the outset of learning, it has been observed that a hippocampal-neocortical binding process occurs similarly to when forming new memories ( Schlichting and Preston, 2015 ). The proposed framework involves the medial temporal lobe (MTL), which is involved in acquiring new information and binds different aspects of an experience into a single memory trace. In contrast, the medial prefrontal cortex (mPFC) integrates this information with the existing knowledge ( Zeithamova and Preston, 2010 ; van Kesteren et al., 2012 ). During consolidation and retrieval, MTL is involved in replaying memories to the neocortex, where they are gradually integrated with existing knowledge and schemas and help retrieve memory traces. During retrieval, the mPFC is thought to use existing knowledge and schemas to guide retrieval and interpretation of memory. This may involve the assimilation of newly acquired information into existing cognitive schemata as opposed to the comparatively slow progression of creating intercortical connections ( Zeithamova and Preston, 2010 ; van Kesteren et al., 2012 , 2016 ).
Medial temporal lobe structures are essential for acquiring new information and necessary for autobiographical (episodic) memory ( Brown et al., 2018 ). The consolidation of autobiographical memories depends on a distributed network of cortical regions. Brain areas such as entorhinal, perirhinal, and parahippocampal cortices are essential for learning new information; however, they have little impact on the recollection of the past ( Squire et al., 2015 ). The hippocampus is a region of the brain that forms episodic memories by linking multiple events to create meaningful experiences ( Cooper and Ritchey, 2019 ). It receives information from all areas of the association cortex and cingulate cortex, subcortical regions via the fornix, as well as signals originating within its entorhinal cortex (EC) and amygdala regarding emotionally laden or potentially hazardous stimuli ( Sorensen, 2009 ). Such widespread connectivity facilitates the construction of an accurate narrative underpinning each remembered episode, transforming short-term into long-term recollections ( Richter-Levin and Akirav, 2003 ).
Researchers have yet to establish a consensus regarding where semantic memory information is localized within the brain ( Roldan-Valadez et al., 2012 ). Some proponents contend that such knowledge is lodged within perceptual and motor systems, triggered when we initially associate with a given object. This point of view is supported by studies highlighting how neural activity occurs initially in the occipital cortex, followed by left temporal lobe involvement during processing and pertinent contributions to word selection/retrieval via activation of left inferior frontal cortices ( Patterson et al., 2007 ). Moreover, research indicates elevated levels of fusiform gyrus engagement (a ventral surface region encompassing both temporal lobes) occurring concomitantly with verbal comprehension initiatives, including reading and naming tasks ( Patterson et al., 2007 ).
Research suggests that the hippocampus is needed for a few years after learning to support semantic memory (factual information), yet, it is not needed for the long term ( Squire et al., 2015 ). However, some forms of memory remain dependent on the hippocampus, such as the retrieval of spatial memory ( Wiltgen et al., 2010 ). Similarly, the Multiple-trace theory ( Moscovitch et al., 2006 ), also known as the transformation hypothesis ( Winocur and Moscovitch, 2011 ), posits that hippocampal engagement is necessary for memories that retain contextual detail such as episodic memories. Consolidation of memories into the neocortex is theorized to involve a loss of specific finer details, such as temporal and spatial information, in addition to contextual elements. This transition ultimately results in an evolution from episodic memory toward semantic memory, which consists mainly of gist-based facts ( Moscovitch et al., 2006 ).
Sleep and memory consolidation
Sleep is an essential physiological process crucial to memory consolidation ( Siegel, 2001 ). Sleep is divided into two stages: Non-rapid Eye Movement (NREM) sleep and Rapid Eye Movement (REM) sleep. NREM sleep is divided into three stages: N1, N2, and N3 (AKA Slow Wave Sleep or SWS) ( Rasch and Born, 2013 ). Each stage displays unique oscillatory patterns and phenomena responsible for consolidating memories in distinct ways. The first stage, or N1 sleep, is when an individual transitions between wakefulness and sleep. This type of sleep is characterized by low-amplitude, mixed-frequency brain activity. N1 sleep is responsible for the initial encoding of memories ( Rasch and Born, 2013 ). The second stage, or N2 sleep, is characterized by the occurrence of distinct sleep spindles and K-complexes in EEG. N2 is responsible for the consolidation of declarative memories ( Marshall and Born, 2007 ). The third stage of sleep N3, also known as slow wave sleep (SWS), is characterized by low-frequency brain activity, slow oscillations, and high amplitude. The slow oscillations which define the deepest stage of sleep are trademark rhythms of NREM sleep. These slow oscillations are delta waves combined to indicate slow wave activity (SWA), which is implicated in memory consolidation ( Tononi and Cirelli, 2003 ; Stickgold, 2005 ; Kim et al., 2019 ). Sleep spindles are another trademark defining NREM sleep ( Stickgold, 2005 ). Ripples are high-frequency bursts, and when combined with irregularly occurring sharp waves (high amplitude), they form the sharp-wave ripple (SWR). These spindles and the SWRs coordinate the reactivation and redistribution of hippocampus-dependent memories to neocortical sites ( Ngo et al., 2020 ; Girardeau and Lopes-dos-Santos, 2021 ). The third stage is also responsible for the consolidation of procedural memories, such as habits and motor skills ( Diekelmann and Born, 2010 ). During SWS, there is minimal cholinergic activity and intermediate noradrenergic activity ( Datta and MacLean, 2007 ).
Finally, the fourth stage of sleep is REM sleep, characterized by phasic REMs and muscle atonia ( Reyes-Resina et al., 2021 ). During REM sleep, there is high cholinergic activity, serotonergic and noradrenergic activity are at a minimum, and high theta activity ( Datta and MacLean, 2007 ). REM sleep is also characterized by local increases in plasticity-related immediate-early gene activity, which might favor the subsequent synaptic consolidation of memories in the cortex ( Ribeiro, 2007 ; Diekelmann and Born, 2010 ; Reyes-Resina et al., 2021 ). The fourth stage of sleep is responsible for the consolidation of emotional memories and the integration of newly acquired memories into existing knowledge structures ( Rasch and Born, 2013 ). Studies indicate that the cholinergic system plays an imperative role in modifying these processes by toggling the entire thalamo-cortico-hippocampal network between distinct modes, namely high Ach encoding mode during active wakefulness and REM sleep and low Ach consolidation mode during quiet wakefulness and NREM sleep ( Bergmann and Staresina, 2017 ; Li et al., 2020 ). Consequently, improving neocortical hippocampal communication results in efficient memory encoding/synaptic plasticity, whereas hippocampo-neocortical interactions favor better systemic memory consolidation ( Diekelmann and Born, 2010 ).
The dual process hypothesis of memory consolidation posits that SWS facilitates declarative, hippocampus-dependent memory, whereas REM sleep facilitates non-declarative hippocampus-independent memory ( Maquet, 2001 ; Diekelmann and Born, 2010 ). On the other hand, the sequential hypothesis states that different sleep stages play a sequential role in memory consolidation. Memories are encoded during wakefulness, consolidated during NREM sleep, and further processed and integrated during REM sleep ( Rasch and Born, 2013 ). However, there is evidence present that contradicts the sequential hypothesis. A study by Goerke et al. (2013) found that declarative memories can be consolidated during REM sleep, suggesting that the relationship between sleep stages and memory consolidation is much more complex than a sequential model. Moreover, other studies indicate the importance of coordinating specific sleep phases with learning moments for optimal memory retention. This indicates that the timing of sleep has more influence than the specific sleep stages ( Gais et al., 2006 ). The active system consolidation theory suggests that an active consolidation process results from the selective reactivation of memories during sleep; the brain selectively reactivates newly encoded memories during sleep, which enhances and integrates them into the network of pre-existing long-term memories ( Born et al., 2006 ; Howard et al., 2022 ). Research has suggested that slow-wave sleep (SWS) and rapid eye movement (REM) sleep have complementary roles in memory consolidation. Declarative and non-declarative memories benefiting differently depending on which sleep stage they rely on ( Bergmann and Staresina, 2017 ). Specifically, during SWS, the brain actively reactivates and reorganizes hippocampo-neocortical memory traces as part of system consolidation. Following this, REM sleep is crucial for stabilizing these reactivated memory traces through synaptic consolidation. While SWS may initiate early plastic processes in hippocampo-neocortical memory traces by “tagging” relevant neocortico-neocortical synapses for later consolidation ( Frey and Morris, 1998 ), long-term plasticity requires subsequent REM sleep ( Rasch and Born, 2007 , 2013 ).
The active system consolidation hypothesis is not the only mechanism proposed for memory consolidation during sleep. The synaptic homeostasis hypothesis proposes that sleep is necessary for restoring synaptic homeostasis, which is challenged by synaptic strengthening triggered by learning during wake and synaptogenesis during development ( Tononi and Cirelli, 2014 ). The synaptic homeostasis hypothesis assumes consolidation is a by-product of the global synaptic downscaling during sleep ( Puentes-Mestril and Aton, 2017 ). The two models are not mutually exclusive, and the hypothesized processes probably act in concert to optimize the memory function of sleep ( Diekelmann and Born, 2010 ).
Non-rapid eye movement sleep plays an essential role in the systems consolidation of memories, with evidence showing that different oscillations are involved in this process ( Düzel et al., 2010 ). With an oscillatory sequence initiated by a slow frontal cortex oscillation (0.5–1 Hz) traveling to the medial temporal lobe and followed by a sharp-wave ripple (SWR) in the hippocampus (100–200 Hz). Replay activity of memories can be measured during this oscillatory sequence across various regions, including the motor cortex and visual cortex ( Ji and Wilson, 2006 ; Eichenlaub et al., 2020 ). Replay activity of memory refers to the phenomenon where the hippocampus replays previously experienced events during sharp wave ripples (SWRs) and theta oscillations ( Zielinski et al., 2018 ). During SWRs, short, transient bursts of high-frequency oscillations occur in the hippocampus. During theta oscillations, hippocampal spikes are ordered according to the locations of their place fields during behavior. These sequential activities are thought to play a role in memory consolidation and retrieval ( Zielinski et al., 2018 ). The paper by Zielinski et al. (2018) suggests that coordinated hippocampal-prefrontal representations during replay and theta sequences play complementary and overlapping roles at different stages in learning, supporting memory encoding and retrieval, deliberative decision-making, planning, and guiding future actions.
Additionally, the high-frequency oscillations of SWR reactivate groups of neurons attributed to spatial information encoding to align synchronized activity across an array of neural structures, which results in distributed memory creation ( Swanson et al., 2020 ; Girardeau and Lopes-dos-Santos, 2021 ). Parallel to this process is slow oscillation or slow-wave activity within cortical regions, which reflects synced neural firing and allows regulation of synaptic weights, which is in accordance with the synaptic homeostasis hypothesis (SHY). The SHY posits that downscaling synaptic strengths help incorporate new memories by avoiding saturation of resources during extended periods–features validated by discoveries where prolonged wakefulness boosts amplitude while it diminishes during stretches of enhanced sleep ( Girardeau and Lopes-dos-Santos, 2021 ).
During REM sleep, the brain experiences “paradoxical” sleep due to the similarity in activity to wakefulness. This stage plays a significant role in memory processing. Theta oscillations which are dominant during REM sleep, are primarily observed in the hippocampus, and these are involved in memory consolidation ( Landmann et al., 2014 ). There has been evidence of coherence between theta oscillations in the hippocampus, medial frontal cortex, and amygdala, which support their involvement in memory consolidation ( Popa et al., 2010 ). During REM sleep, phasic events such as ponto-geniculo-occipital waves originating from the brainstem coordinate activity across various brain structures and may contribute to memory consolidation processes ( Rasch and Born, 2013 ). Research has suggested that sleep-associated consolidation may be mediated by the degree of overlap between new and already known material whereby, if the acquired information is similar to the information one has learned, it is more easily consolidated during sleep ( Tamminen et al., 2010 ; Sobczak, 2017 ).
In conclusion, understanding more about how the brains cycle through different stages of sleep, including specific wave patterns, offers valuable insight into the ability to store memories effectively. While NREM sleep is associated with SWRs and slow oscillations, facilitating memory consolidation and synaptic downscaling, REM sleep, characterized by theta oscillations and phasic events, contributes to memory reconsolidation and the coordination of activity across brain regions. By exploring the interactions between sleep stages, oscillations, and memory processes, one may learn more about how sleep impacts brain function and cognition in greater detail.
Century has passed since we addressed memory, and several notable findings have moved from bench-to-bedside research. Several cross-talks between multidiscipline have been encouraged. Nevertheless, further research is needed into neurobiological mechanisms of non-declarative memory, such as conditioning ( Gallistel and Balsam, 2014 ). Modern research indicates that structural change that encodes information is likely at the level of the synapse, and the computational mechanisms are implemented at the level of neural circuitry. However, it also suggests that intracellular mechanisms realized at the molecular level, such as micro RNAs, should not be discounted as potential mechanisms. However, further research is needed to study the molecular and structural changes brought on by implicit memory ( Gallistel and Balsam, 2014 ).
The contribution of non-human animal studies toward our understanding of memory processes cannot be understated; hence recognizing their value is vital for moving forward. While this paper predominantly focused on cognitive neuroscience perspectives, some articles cited within this paper were sourced from non-human animal studies providing fundamental groundwork and identification of critical mechanisms relevant to human memories. A need persists for further investigation—primarily with humans—which can validate existing findings from non-human animals. Moving forward, it is prudent for researchers to bridge the gap between animal and human investigations done while exploring parallels and exploring unique aspects of human memory processes. By integrating findings from both domains, one can gain a more comprehensive understanding of the complexities of memory and its underlying neural mechanisms. Such investigations will broaden the horizon of our memory process and answer the complex nature of memory storage.
This paper attempted to provide an overview and summarize memory and its processes. The paper focused on bringing the cognitive neuroscience perspective on memory and its processes. This may provide the readers with the understanding, limitations, and research perspectives of memory mechanisms.
Data availability statement
Author contributions.
SS and MKA: conceptualization, framework, and manuscript writing. AK: review and editing of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We gratefully thank students and Indian Institute of Technology Roorkee (IITR) office staff for their conditional and unconditional support. We also thank the Memory and Anxiety Research Group (MARG), IIT Roorkee for its constant support.
Funding Statement
MKA was supported by the F.I.G. grant (IITR/SRIC/2741). The funding agency had no role in the preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
- Abraham W. C., Jones O. D., Glanzman D. L. (2019). Is plasticity of synapses the mechanism of long-term memory storage? NPJ Sci. Learn. 4 : 9 . 10.1038/s41539-019-0048-y [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Asthana M. K., Brunhuber B., Mühlberger A., Reif A., Schneider S., Herrmann M. J. (2015). Preventing the return of fear using reconsolidation update mechanisms depends on the met-allele of the brain derived neurotrophic factor val66met polymorphism. Int. J. Neuropsychopharmacol . 19 : yv137 . 10.1093/ijnp/pyv137 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Atkinson R. C., Shiffrin R. M. (1968). “ Human memory: a proposed system and its control processes ,” in Psychology of learning and motivation , Vol. 2 eds Spence K. W., Spence J. T. (Amsterdam: Elsevier; ), 89–195. 10.1016/S0079-7421(08)60422-3 [ CrossRef ] [ Google Scholar ]
- Avraham G., Leib R., Pressman A., Simo L. S., Karniel A., Shmuelof L., et al. (2017). State-based delay representation and its transfer from a game of pong to reaching and tracking. Eneuro 4 ENEURO.179–ENEURO.117. 10.1523/eneuro.0179-17.2017 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Awh E., Jonides J., Smith E. E., Schumacher E. H., Koeppe R. A., Katz S. (1996). Dissociation of storage and rehearsal in verbal working memory: evidence From Positron Emission Tomography. Psychol. Sci. 7 25–31. 10.1111/j.1467-9280.1996.tb00662.x [ CrossRef ] [ Google Scholar ]
- Baddeley A. (2000). The episodic buffer: a new component of working memory? Trends Cogn. Sci. 4 417–423. 10.1016/S1364-6613(00)01538-2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baddeley A. (2003). Working memory: looking back and looking forward. Nat. Rev. Neurosci. 4 829–839. 10.1038/nrn1201 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baddeley A. D. (1986). Working memory. Oxford: Oxford University Press. [ Google Scholar ]
- Baddeley A. D., Allen R. J., Hitch G. J. (2011). Binding in visual working memory: the role of the episodic buffer. Neuropsychologia 49 1393–1400. 10.1016/j.neuropsychologia.2010.12.042 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baddeley A. D., Hitch G. (1974). “ Working memory ,” in Psychology of learning and motivation , Vol. 8 ed. Bower G. A. (Amsterdam: Elsevier; ), 47–89. 10.1016/S0079-7421(08)60452-1 [ CrossRef ] [ Google Scholar ]
- Baddeley A. D., Logie R. H. (1999). “ Working memory: the multiple-component model ,” in Models of working memory , 1st Edn, eds Miyake A., Shah P. (Cambridge: Cambridge University Press; ), 28–61. 10.1017/CBO9781139174909.005 [ CrossRef ] [ Google Scholar ]
- Baddeley A., Allen R. J., Hitch G. J. (2010). Investigating the episodic buffer. Psychol. Belgica 50 223 . 10.5334/pb-50-3-4-223 [ CrossRef ] [ Google Scholar ]
- Badre D. (2008). Cognitive control, hierarchy, and the Rostro–caudal organization of the frontal lobes. Trends Cogn. Sci. 12 193–200. 10.1016/j.tics.2008.02.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Badre D., Nee D. E. (2018). Frontal cortex and the hierarchical control of behavior. Trends Cogn. Sci. 22 170–188. 10.1016/j.tics.2017.11.005 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bailey C. H., Kandel E. R., Harris K. M. (2015). Structural components of synaptic plasticity and memory consolidation. Cold Spring Harb. Perspect. Biol. 7 : a021758 . 10.1101/cshperspect.a021758 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bellfy L., Kwapis J. L. (2020). Molecular mechanisms of reconsolidation-dependent memory updating. Int. J. Mol. Sci. 21 : 6580 . 10.3390/ijms21186580 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bergmann T. O., Staresina B. P. (2017). “ Neuronal oscillations and reactivation subserving memory consolidation ,” in Cognitive neuroscience of memory consolidation , eds Axmacher N., Rasch B. (Cham: Springer; ), 185–207. 10.1007/978-3-319-45066-7_12 [ CrossRef ] [ Google Scholar ]
- Berlingeri M., Bottini G., Basilico S., Silani G., Zanardi G., Sberna M., et al. (2008). Anatomy of the episodic buffer: a Voxel-based morphometry study in patients with dementia. Behav. Neurol. 19 29–34. 10.1155/2008/828937 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bermudez-Rattoni F. (2010). Is memory consolidation a multiple-circuit system? Proc. Natl. Acad. Sci. U.S.A. 107 8051–8052. 10.1073/pnas.1003434107 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Binder J. R., Desai R. H. (2011). The neurobiology of semantic memory. Trends Cogn. Sci. 15 527–536. 10.1016/j.tics.2011.10.001 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Binney R. J., Hoffman P., Lambon Ralph M. A. (2016). Mapping the multiple graded contributions of the anterior temporal lobe representational hub to abstract and social concepts: evidence from distortion-corrected fmri. Cereb. Cortex 26 4227–4241. 10.1093/cercor/bhw260 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bliss T. V., Collingridge G. L. (1993). A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361 31–39. 10.1038/361031a0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Blumenfeld R. S., Ranganath C. (2007). Prefrontal cortex and long-term memory encoding: an integrative review of findings from Neuropsychology and Neuroimaging. Neuroscientist 13 280–291. 10.1177/1073858407299290 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Boran E., Fedele T., Klaver P., Hilfiker P., Stieglitz L., Grunwald T., et al. (2019). Persistent hippocampal neural firing and hippocampal-cortical coupling predict verbal working memory load. Sci. Adv. 5 : eaav3687 . 10.1126/sciadv.aav3687 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Born J., Rasch B., Gais S. (2006). Sleep to remember. Neuroscientist 12 410–424. 10.1177/1073858406292647 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Braver T. S., Gray J. R., Burgess G. C. (2008). “ Explaining the many varieties of working memory variation: dual mechanisms of cognitive control ,” in Variation in working memory , 1st Edn, eds Conway A., Jarrold C., Kane M., Miyake A., Towse J. (New York, NY: Oxford University Press; ), 76–106. 10.1093/acprof:oso/9780195168648.003.0004 [ CrossRef ] [ Google Scholar ]
- Brown T. I., Rissman J., Chow T. E., Uncapher M. R., Wagner A. D. (2018). Differential medial temporal lobe and parietal cortical contributions to real-world autobiographical episodic and autobiographical semantic memory. Sci. Rep. 8 : 6190 . 10.1038/s41598-018-24549-y [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Buchsbaum B. R. (2010). Neural basis of working memory. Encyclopedia of Behavioral Neuroscience 334–341. 10.1016/b978-0-08-045396-5.00161-5 [ CrossRef ] [ Google Scholar ]
- Buchsbaum B. R., Hickok G., Humphries C. (2001). Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cogn. Sci. 25 663–678. 10.1207/s15516709cog2505_2 [ CrossRef ] [ Google Scholar ]
- Buiatti T., Mussoni A., Toraldo A., Skrap M., Shallice T. (2011). Two qualitatively different impairments in making rotation operations. Cortex 47 166–179. 10.1016/j.cortex.2009.10.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Burbaud P., Camus O., Caillé J., Biolac B., Allard M. (1999). Influence of individual strategies on brain activation patterns during cognitive tasks. J. Neuroradiol. 26 59–65. [ PubMed ] [ Google Scholar ]
- Camina E., Güell F. (2017). The neuroanatomical, neurophysiological and psychological basis of memory: current models and their origins. Front. Pharmacol. 8 : 438 . 10.3389/fphar.2017.00438 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Caplan D., Waters G. (2005). The relationship between age, processing speed, working memory capacity, and language comprehension. Memory 13 403–413. 10.1080/09658210344000459 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chein J. M., Ravizza S. M., Fiez J. A. (2003). Using neuroimaging to evaluate models of working memory and their implications for language processing. J. Neurolinguist. 16 315–339. 10.1016/s0911-6044(03)00021-6 [ CrossRef ] [ Google Scholar ]
- Clawson B. C., Pickup E. J., Ensing A., Geneseo L., Shaver J., Gonzalez-Amoretti J., et al. (2021). Causal role for sleep-dependent reactivation of learning-activated sensory ensembles for fear memory consolidation . Nat. Commun . 12 , 1–13. 10.1038/s41467-021-21471-2 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cohen M. S., Kosslyn S. M., Breiter H. C., DiGirolamo G. J., Thompson W. L., Anderson A. K., et al. (1996). Changes in cortical activity during mental rotation a mapping study using functional MRI. Brain 119 89–100. 10.1093/brain/119.1.89 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cooper R. A., Ritchey M. (2019). Progression from feature-specific brain activity to hippocampal binding during episodic encoding. J. Neurosci. 40 1701–1709. 10.1523/jneurosci.1971-19.2019 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Curtis C. E., Sprague T. C. (2021). Persistent activity during working memory from front to back. Front. Neural Circ. 15 : 696060 . 10.3389/fncir.2021.696060 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- D’Esposito M. (2007). From cognitive to neural models of working memory. Philos. Trans. R. Soc. B Biol. Sci. 362 761–772. 10.1098/rstb.2007.2086 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- D’Esposito M., Postle B. R. (2015). The cognitive neuroscience of working memory. Annu. Rev. Psychol. 66 115–142. 10.1146/annurev-psych-010814-015031 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Daselaar S. M. (2009). Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Front. Hum. Neurosci. 3 : 13 . 10.3389/neuro.09.013.2009 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Datta S., MacLean R. R. (2007). Neurobiological mechanisms for the regulation of mammalian sleep–wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci. Biobehav. Rev. 31 775–824. 10.1016/j.neubiorev.2007.02.004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Davis S. W., Wing E. A., Cabeza R. (2018). Contributions of the ventral parietal cortex to declarative memory. Handb. Clin. Neurol. 151 525–553. 10.1016/b978-0-444-63622-5.00027-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Derner M., Dehnen G., Chaieb L., Reber T. P., Borger V., Surges R., et al. (2020). Patterns of single-neuron activity during associative recognition memory in the human medial temporal lobe. Neuroimage 221 : 117214 . 10.1016/j.neuroimage.2020.117214 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dew I. T. Z., Cabeza R. (2011). The porous boundaries between explicit and implicit memory: behavioral and neural evidence. Ann. N. Y. Acad. Sci. 1224 174–190. 10.1111/j.1749-6632.2010.05946.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Diekelmann S., Born J. (2010). The memory function of sleep. Nat. Rev. Neurosci. 11 114–126. 10.1038/nrn2762 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dudai Y. (2002). Molecular bases of long-term memories: a question of persistence. Curr. Opin. Neurobiol. 12 211–216. 10.1016/s0959-4388(02)00305-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dudai Y., Karni A., Born J. (2015). The consolidation and transformation of memory. Neuron 88 20–32. 10.1016/j.neuron.2015.09.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dunsmoor J. E., Murty V. P., Davachi L., Phelps E. A. (2015). Emotional learning selectively and retroactively strengthens memories for related events. Nature 520 345–348. 10.1038/nature14106 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Düzel E., Penny W. D., Burgess N. (2010). Brain oscillations and memory. Curr. Opin. Neurobiol. 20 143–149. 10.1016/j.conb.2010.01.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eichenbaum H., Cohen N. J. (2014). Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron 83 764–770. 10.1016/j.neuron.2014.07.032 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eichenlaub J.-B., Jarosiewicz B., Saab J., Franco B., Kelemen J., Halgren E., et al. (2020). Replay of learned neural firing sequences during rest in human motor cortex. Cell Rep. 31 : 107581 . 10.1016/j.celrep.2020.107581 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Emch M., von Bastian C. C., Koch K. (2019). Neural correlates of verbal working memory: an fMRI meta-analysis. Front. Hum. Neurosci. 13 : 180 . 10.3389/fnhum.2019.00180 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Frankland P. W., Bontempi B. (2005). The organization of recent and remote memories. Nature Rev. Neurosci. 6 119–130. 10.1038/nrn1607 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Frey U., Morris R. G. M. (1998). Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 21 181–188. 10.1016/s0166-2236(97)01189-2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Friedman N. P., Robbins T. W. (2022). The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 47 72–89. 10.1038/s41386-021-01132-0 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Funahashi S. (2017). Working memory in the prefrontal cortex. Brain Sci. 7 : 49 . 10.3390/brainsci7050049 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gais S., Lucas B., Born J. (2006). Sleep after learning AIDS memory recall. Learn. Mem. 13 259–262. 10.1101/lm.132106 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gallistel C. R., Balsam P. D. (2014). Time to rethink the neural mechanisms of learning and memory. Neurobiol. Learn. Mem. 108 136–144. 10.1016/j.nlm.2013.11.019 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ge Y., Dong Z., Bagot R. C., Howland J. G., Phillips A. G., Wong T. P., et al. (2010). Hippocampal long-term depression is required for the consolidation of Spatial Memory. Proc. Natl Acad. Sci. U.S.A. 107 16697–16702. 10.1073/pnas.1008200107 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gelastopoulos A., Whittington M. A., Kopell N. J. (2019). Parietal low beta rhythm provides a dynamical substrate for a working memory buffer. Proc. Natl Acad. Sci. U.S.A. 116 16613–16620. 10.1073/pnas.1902305116 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Genzel L., Wixted J. T. (2017). “ Cellular and systems consolidation of declarative memory ,” in Cognitive neuroscience of memory consolidation , eds Axmacher N., Rasch B. (Berlin: Springer International Publishing; ), 3–16. 10.1007/978-3-319-45066-7_1 [ CrossRef ] [ Google Scholar ]
- Ghaleh M., Lacey E. H., Fama M. E., Anbari Z., DeMarco A. T., Turkeltaub P. E. (2019). Dissociable mechanisms of verbal working memory revealed through multivariate lesion mapping. Cereb. Cortex 30 2542–2554. 10.1093/cercor/bhz259 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gilboa A., Moscovitch M. (2021). No consolidation without representation: correspondence between neural and psychological representations in recent and remote memory. Neuron 109 2239–2255. 10.1016/j.neuron.2021.04.025 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Girardeau G., Lopes-dos-Santos V. (2021). Brain neural patterns and the memory function of sleep. Science 374 560–564. 10.1126/science.abi8370 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Goedert K. M., Willingham D. B. (2002). Patterns of interference in sequence learning and prism adaptation inconsistent with the consolidation hypothesis. Learn. Mem. 9 279–292. 10.1101/lm.50102 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Goerke M., Cohrs S., Rodenbeck A., Grittner U., Sommer W., Kunz D. (2013). Declarative memory consolidation during the first night in a sleep lab: the role of REM sleep and Cortisol. Psychoneuroendocrinology 38 1102–1111. 10.1016/j.psyneuen.2012.10.019 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Graves W. W., Grabowski T. J., Mehta S., Gupta P. (2008). The left posterior superior temporal gyrus participates specifically in accessing lexical phonology. J. Cogn. Neurosci. 20 1698–1710. 10.1162/jocn.2008.20113 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hamann S. (2001). Cognitive and neural mechanisms of emotional memory. Trends Cogn. Sci. 5 394–400. 10.1016/S1364-6613(00)01707-1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Henson R. N., Rugg M. D., Shallice T., Josephs O., Dolan R. J. (1999). Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J. Neurosci. 19 3962–3972. 10.1523/jneurosci.19-10-03962.1999 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Herry C., Johansen J. P. (2014). Encoding of fear learning and memory in distributed neuronal circuits. Nat. Neurosci. 17 1644–1654. 10.1038/nn.3869 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hitch G. J., Allen R. J., Baddeley A. D. (2019). Attention and binding in visual working memory: two forms of attention and two kinds of buffer storage. Attent. Percept. Psychophys. 82 280–293. 10.3758/s13414-019-01837-x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Howard M. D., Skorheim S. W., Pilly P. K. (2022). A model of bi-directional interactions between complementary learning systems for memory consolidation of sequential experiences. Front. Syst. Neurosci. 16 : 972235 . 10.3389/fnsys.2022.972235 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hutchinson J. B., Uncapher M. R., Wagner A. D. (2009). Posterior parietal cortex and episodic retrieval: convergent and divergent effects of attention and memory. Learn. Mem. 16 343–356. 10.1101/lm.919109 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ikkai A., Curtis C. E. (2011). Common neural mechanisms supporting spatial working memory, attention and motor intention. Neuropsychologia 49 1428–1434. 10.1016/j.neuropsychologia.2010.12.020 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ito M., Kuroiwa J., Miyake S. (2003). A neural network model of memory system using hippocampus. Electron. Commun. Japan 86 86–97. 10.1002/ecjc.1010 [ CrossRef ] [ Google Scholar ]
- Ivanco T. L. (2015). Long-term potentiation and long-term depression. International Encyclopedia of the Social & Behavioral Sciences 14 , 358–365. 10.1016/b978-0-08-097086-8.55034-x [ CrossRef ] [ Google Scholar ]
- Jerde T. A., Curtis C. E. (2013). Maps of space in human frontoparietal cortex. J. Physiol. 107 510–516. 10.1016/j.jphysparis.2013.04.002 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ji D., Wilson M. A. (2006). Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 10 100–107. 10.1038/nn1825 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Josselyn S. A., Köhler S., Frankland P. W. (2015). Finding the engram. Nat. Rev. Neurosci. 16 521–534. 10.1038/nrn4000 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jung J., Lambon Ralph M. A., Jackson R. L. (2022). Subregions of DLPFC display graded yet distinct structural and functional connectivity. J. Neurosci. 42 3241–3252. 10.1523/jneurosci.1216-21.2022 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kaleem A., Hoessli D. C., Haq I., Walker-Nasir E., Butt A., Iqbal Z., et al. (2011). CREB in long-term potentiation in hippocampus: role of post-translational modifications-studies in silico. J. Cell. Biochem. 112 138–146. 10.1002/jcb.22909 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kamiński J., Rutishauser U. (2019). Between persistently active and activity-silent frameworks: novel vistas on the cellular basis of working memory. Ann. N. Y. Acad. Sci. 1464 64–75. 10.1111/nyas.14213 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kamiński J., Sullivan S., Chung J. M., Ross I. B., Mamelak A. N., Rutishauser U. (2017). Persistently active neurons in human medial frontal and medial temporal lobe support working memory. Nat. Neurosci. 20 590–601. 10.1038/nn.4509 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kandel E. R., Mack S. (2013). Principles of neural science. New York, NY: McGraw-Hill Medical. [ Google Scholar ]
- Kandel E. R., Dudai Y., Mayford M. R. (2014). The molecular and systems biology of memory. Cell 157 163–186. 10.1016/j.cell.2014.03.001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Karni A., Meyer G., Rey-Hipolito C., Jezzard P., Adams M. M., Turner R., et al. (1998). The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc. Natl. Acad. Sci. U.S.A. 95 861–868. 10.1073/pnas.95.3.861 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kerzel D., Burra N. (2020). Capture by context elements, not attentional suppression of distractors, explains the PD with small search displays. J. Cogn. Neurosci. 32 1170–1183. 10.1162/jocn_a_01535 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kesner R. P. (2017). Memory neurobiology?. Reference Module in Neuroscience and Biobehavioral Psychology 1–12. 10.1016/b978-0-12-809324-5.03089-3 [ CrossRef ] [ Google Scholar ]
- Kim J., Gulati T., Ganguly K. (2019). Competing roles of slow oscillations and delta waves in memory consolidation versus forgetting. Cell 179 514–526.e13. 10.1016/j.cell.2019.08.040 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Koenigs M., Acheson D. J., Barbey A. K., Solomon J., Postle B. R., Grafman J. (2011). Areas of left perisylvian cortex mediate auditory–verbal short-term memory. Neuropsychologia 49 3612–3619. 10.1016/j.neuropsychologia.2011.09.013 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kosslyn S. M., Thompson W. L., Alpert N. M. (1997). Neural Systems shared by visual imagery and visual perception: a positron emission tomography study. Neuroimage 6 320–334. 10.1006/nimg.1997.0295 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kuhnke P., Kiefer M., Hartwigsen G. (2020). Task-dependent recruitment of modality-specific and multimodal regions during conceptual processing. Cereb. Cortex 30 3938–3959. 10.1093/cercor/bhaa010 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Landmann N., Kuhn M., Piosczyk H., Feige B., Baglioni C., Spiegelhalder K., et al. (2014). The reorganisation of memory during sleep. Sleep Med. Rev. 18 531–541. 10.1016/j.smrv.2014.03.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Langille J. J. (2019). Remembering to forget: a dual role for sleep oscillations in memory consolidation and forgetting. Front. Cell. Neurosci. 13 : 71 . 10.3389/fncel.2019.00071 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Langille J. J., Brown R. E. (2018). The synaptic theory of memory: a historical survey and reconciliation of recent opposition. Front. Syst. Neurosci. 12 : 52 . 10.3389/fnsys.2018.00052 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lee H., Chun M. M., Kuhl B. A. (2016). Lower parietal encoding activation is associated with sharper information and Better Memory. Cereb. Cortex 27 , 2486–2499. 10.1093/cercor/bhw097 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li M., Lu S., Wu Y., Zhong N. (2010). “ Functional segregation of memory encoding and retrieval in the prefrontal cortex ,” in Proceedings of the IEEE/ICME International Conference on Complex Medical Engineering , (Gold Coast, QLD: ), 91–95. 10.1109/iccme.2010.5558865 [ CrossRef ] [ Google Scholar ]
- Li Q., Song J.-L., Li S.-H., Westover M. B., Zhang R. (2020). Effects of cholinergic neuromodulation on thalamocortical rhythms during NREM sleep: a model study. Front. Comput. Neurosci. 13 : 100 . 10.3389/fncom.2019.00100 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lisman J. E., Grace A. A. (2005). The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 46 703–713. 10.1016/j.neuron.2005.05.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liu J., Zhang H., Yu T., Ren L., Ni D., Yang Q., et al. (2021). Transformative neural representations support long-term episodic memory. Sci. Adv. 7 : eabg9715 . 10.1126/sciadv.abg9715 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Luscher C., Malenka R. C. (2012). NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 4 a005710–a005710. 10.1101/cshperspect.a005710 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lynch M. A. (2004). Long-term potentiation and memory. Physiol. Rev. 84 87–136. 10.1152/physrev.00014.2003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Maass W., Markram H. (2002). Synapses as dynamic memory buffers. Neural Netw. 15 155–161. 10.1016/s0893-6080(01)00144-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mackey W. E., Devinsky O., Doyle W. K., Golfinos J. G., Curtis C. E. (2016). Human parietal cortex lesions impact the precision of spatial working memory. J. Neurophysiol. 116 1049–1054. 10.1152/jn.00380.2016 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Malenka R. C., Bear M. F. (2004). LTP and Ltd. Neuron 44 5–21. 10.1016/j.neuron.2004.09.012 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Malenka R. C., Nicoll R. (1999). Long-term potentiation–a decade of progress? Science 285 1870–1874. 10.1126/science.285.5435.1870 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mank M., Griesbeck O. (2008). Genetically encoded calcium indicators. Chem. Rev. 108 1550–1564. 10.1021/cr078213v [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Maquet P. (2001). The role of sleep in learning and memory. Science 294 1048–1052. 10.1126/science.1062856 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marshall L., Born J. (2007). The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn. Sci. 11 442–450. 10.1016/j.tics.2007.09.001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mayes A. R. (2002). Memory disorders, organic. Encyclopedia of the Human Brain 2 , 759–772. 10.1016/b0-12-227210-2/00199-0 [ CrossRef ] [ Google Scholar ]
- McGaugh J. L. (2000). Memory–a century of consolidation. Science 287 248–251. 10.1126/science.287.5451.248 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mednick S. C., Cai D. J., Shuman T., Anagnostaras S., Wixted J. T. (2011). An opportunistic theory of cellular and systems consolidation. Trends Neurosci. 34 504–514. 10.1016/j.tins.2011.06.003 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Menon V., D’Esposito M. (2021). The role of PFC networks in cognitive control and executive function. Neuropsychopharmacology 47 90–103. 10.1038/s41386-021-01152-w [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Miyake A., Friedman N. P., Rettinger D. A., Shah P., Hegarty M. (2001). How are visuospatial working memory, executive functioning, and spatial abilities related? A latent-variable analysis. J. Exp. Psychol. 130 621–640. 10.1037/0096-3445.130.4.621 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moncada D., Ballarini F., Viola H. (2015). Behavioral tagging: a translation of the synaptic tagging and capture hypothesis. Neural Plast. 2015 1–21. 10.1155/2015/650780 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moro V., Berlucchi G., Lerch J., Tomaiuolo F., Aglioti S. M. (2008). Selective deficit of mental visual imagery with intact primary visual cortex and visual perception. Cortex 44 109–118. 10.1016/j.cortex.2006.06.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moscovitch M., Nadel L., Winocur G., Gilboa A., Rosenbaum R. S. (2006). The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr. Opin. Neurobiol. 16 179–190. 10.1016/j.conb.2006.03.013 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nabavi S., Fox R., Proulx C. D., Lin J. Y., Tsien R. Y., Malinow R. (2014). Engineering A memory with ltd and LTP. Nature 511 348–352. 10.1038/nature13294 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nader K., Hardt O. (2009). A single standard for memory: the case for reconsolidation. Nat. Rev. Neurosci. 10 224–234. 10.1038/nrn2590 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Narayanan N. S., Prabhakaran V., Bunge S. A., Christoff K., Fine E. M., Gabrieli J. D. E. (2005). The role of the prefrontal cortex in the maintenance of verbal working memory: an event-related fMRI analysis. Neuropsychology 19 223–232. 10.1037/0894-4105.19.2.223 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nee D. E., D’Esposito M. (2016). The hierarchical organization of the lateral prefrontal cortex. eLife 5 : e12112 . 10.7554/elife.12112 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ngo H.-V., Fell J., Staresina B. (2020). Sleep spindles mediate hippocampal-neocortical coupling during long-duration ripples. eLife 9 : e57011 . 10.7554/elife.57011 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ober B. A. (2014). Memory, explicit/implicit. Encyclopedia of the Neurological Sciences 2 , 1042–1044. 10.1016/b978-0-12-385157-4.00455-3 [ CrossRef ] [ Google Scholar ]
- Okuda K., Højgaard K., Privitera L., Bayraktar G., Takeuchi T. (2020). Initial memory consolidation and the synaptic tagging and capture hypothesis. Eur. J. Neurosci. 54 6826–6849. 10.1111/ejn.14902 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Orsini C. A., Maren S. (2012). Neural and cellular mechanisms of fear and extinction memory formation. Neurosci. Biobehav. Rev. 36 1773–1802. 10.1016/j.neubiorev.2011.12.014 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Osaka N., Logie R. H., D’Esposito M. (eds) (2007). The cognitive neuroscience of working memory. Oxford: Oxford University Press. [ Google Scholar ]
- Panzeri S., Janotte E., Pequeńo-Zurro A., Bonato J., Bartolozzi C. (2023). Constraints on the design of neuromorphic circuits set by the properties of neural population codes. Neuromorphic Computing Eng. 3 : 012001 . 10.1088/2634-4386/acaf9c [ CrossRef ] [ Google Scholar ]
- Park P., Kang H., Georgiou J., Zhuo M., Kaang B.-K., Collingridge G. L. (2021). Further evidence that CP-ampars are critically involved in synaptic tag and capture at hippocampal CA1 synapses. Mol. Brain 14 : 26 . 10.1186/s13041-021-00737-2 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Park P., Kang H., Sanderson T. M., Bortolotto Z. A., Georgiou J., Zhuo M., et al. (2018). The role of calcium-permeable ampars in long-term potentiation at principal neurons in the rodent hippocampus. Front. Synaptic Neurosci. 10 : 42 . 10.3389/fnsyn.2018.00042 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Patterson K., Nestor P. J., Rogers T. T. (2007). Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 8 976–987. 10.1038/nrn2277 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Paulesu E., Frith C. D., Frackowiak R. S. (1993). The neural correlates of the verbal component of working memory. Nature 362 342–345. 10.1038/362342a0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Perrachione T. K., Ghosh S. S., Ostrovskaya I., Gabrieli J. D., Kovelman I. (2017). Phonological working memory for words and nonwords in cerebral cortex. J. Speech Lang. Hear. Res. 60 1959–1979. 10.1044/2017_jslhr-l-15-0446 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Popa D., Duvarci S., Popescu A. T., Léna C., Paré D. (2010). Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc. Natl. Acad. Sci. U.S.A. 107 6516–6519. 10.1073/pnas.0913016107 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Postle B. R. (2006). Working memory as an emergent property of the mind and brain. Neuroscience 139 23–38. 10.1016/j.neuroscience.2005.06.005 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Postle B. R. (2015). The cognitive neuroscience of visual short-term memory. Curr. Opin. Behav. Sci. 1 40–46. 10.1016/j.cobeha.2014.08.004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Puentes-Mestril C., Aton S. J. (2017). Linking network activity to synaptic plasticity during sleep: hypotheses and recent data. Front. Neural Circ. 11 : 61 . 10.3389/fncir.2017.00061 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Qasim S. E., Mohan U. R., Stein J. M., Jacobs J. (2023). Neuronal activity in the human amygdala and hippocampus enhances emotional memory encoding. Nat. Hum. Behav. 7 754–764. 10.1038/s41562-022-01502-8 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Quentin R., King J.-R., Sallard E., Fishman N., Thompson R., Buch E. R., et al. (2019). Differential brain mechanisms of selection and maintenance of information during working memory. J. Neurosci. 39 3728–3740. 10.1523/jneurosci.2764-18.2019 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rakowska M., Bagrowska P., Lazari A., Navarrete M., Abdellahi M. E., Johansen-Berg H., et al. (2022). Cueing motor memory reactivation during NREM sleep engenders learning-related changes in precuneus and sensorimotor structures . bioRxiv 2022.01.-27.477838. 10.1101/2022.01.27.477838 [ CrossRef ] [ Google Scholar ]
- Rasch B., Born J. (2007). Maintaining memories by reactivation. Curr. Opin. Neurobiol. 17 698–703. 10.1016/j.conb.2007.11.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rasch B., Born J. (2013). About sleep’s role in memory. Physiol. Rev. 93 681–766. 10.1152/physrev.00032.2012 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ratcliffe O., Shapiro K., Staresina B. P. (2022). Fronto-medial theta coordinates posterior maintenance of working memory content. Curr. Biol. 32 2121–2129.e3. 10.1016/j.cub.2022.03.045 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reber P. J. (2008). Cognitive neuroscience of declarative and nondeclarative memory. Hum. Learn. Biol. Brain Neurosci. 139 113–123. 10.1016/s0166-4115(08)10010-3 [ CrossRef ] [ Google Scholar ]
- Redondo R. L., Morris R. G. M. (2011). Making memories last: the synaptic tagging and capture hypothesis. Nat. Rev. Neurosci. 12 17–30. 10.1038/nrn2963 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ren Z., Zhang Y., He H., Feng Q., Bi T., Qiu J. (2019). The different brain mechanisms of object and spatial working memory: voxel-based morphometry and resting-state functional connectivity. Front. Hum. Neurosci. 13 : 248 . 10.3389/fnhum.2019.00248 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Repovs G., Baddeley A. (2006). The multi-component model of working memory: explorations in experimental cognitive psychology. Neuroscience 139 5–21. 10.1016/j.neuroscience.2005.12.061 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reyes-Resina I., Samer S., Kreutz M. R., Oelschlegel A. M. (2021). Molecular mechanisms of memory consolidation that operate during sleep. Front. Mol. Neurosci. 14 : 767384 . 10.3389/fnmol.2021.767384 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ribeiro S. (2007). Novel experience induces persistent sleep-dependent plasticity in the cortex but not in the hippocampus. Front. Neurosci. 1 :43–55. 10.3389/neuro.01.1.1.003.2007 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Richter-Levin G., Akirav I. (2003). Emotional tagging of memory formation—In the search for neural mechanisms. Brain Res. Rev. 43 247–256. 10.1016/j.brainresrev.2003.08.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ritchey M., Dolcos F., Cabeza R. (2008). Role of amygdala connectivity in the persistence of emotional memories over time: an event-related fmri investigation. Cereb. Cortex 18 2494–2504. 10.1093/cercor/bhm262 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Robin J., Moscovitch M. (2017). Details, gist and schema: hippocampal–neocortical interactions underlying recent and remote episodic and spatial memory. Curr. Opin. Behav. Sci. 17 114–123. 10.1016/j.cobeha.2017.07.016 [ CrossRef ] [ Google Scholar ]
- Robins S. K. (2020). Stable engrams and neural dynamics. Philos. Sci. 87 1130–1139. 10.1086/710624 [ CrossRef ] [ Google Scholar ]
- Roediger H. L., Butler A. C. (2011). The critical role of retrieval practice in long-term retention. Trends Cogn. Sci. 15 20–27. 10.1016/j.tics.2010.09.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Roldan-Valadez E., García-Lázaro H., Lara-Romero R., Ramirez-Carmona R. (2012). Neuroanatomy of episodic and semantic memory in humans: a brief review of neuroimaging studies. Neurol. India 60 : 613 . 10.4103/0028-3886.105196 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Saffran N. M. (1997). Language and auditory-verbal short-term memory impairments: evidence for common underlying processes. Cogn. Neuropsychol. 14 641–682. 10.1080/026432997381402 [ CrossRef ] [ Google Scholar ]
- Salvato G., Peviani V., Scarpa P., Francione S., Castana L., Gallace A., et al. (2021). Investigating visuo-spatial neglect and visual extinction during intracranial electrical stimulations: the role of the right inferior parietal cortex. Neuropsychologia 162 : 108049 . 10.1016/j.neuropsychologia.2021.108049 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sarnthein J., Petsche H., Rappelsberger P., Shaw G. L., von Stein A. (1998). Synchronization between prefrontal and posterior association cortex during human working memory. Proc. Natl. Acad. Sci. U.S.A. 95 7092–7096. 10.1073/pnas.95.12.7092 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schiller D., Monfils M.-H., Raio C. M., Johnson D. C., LeDoux J. E., Phelps E. A. (2009). Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463 49–53. 10.1038/nature08637 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schlichting M. L., Preston A. R. (2015). Memory integration: neural mechanisms and implications for behavior. Curr. Opin. Behav. Sci. 1 1–8. 10.1016/j.cobeha.2014 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sellers K. K., Yu C., Zhou Z. C., Stitt I., Li Y., Radtke-Schuller S., et al. (2016). Oscillatory dynamics in the frontoparietal attention network during sustained attention in the ferret. Cell Rep. 16 2864–2874. 10.1016/j.celrep.2016.08.055 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sendi M. S., Kanta V., Inman C. S., Manns J. R., Hamann S., Gross R. E., et al. (2020). “ Amygdala stimulation leads to functional network connectivity state transitions in the hippocampus ,” in Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC) , (Montreal, QC: ). 10.1109/embc44109.2020.9176742 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Serences J. T. (2016). Neural mechanisms of information storage in visual short-term memory. Vis. Res. 128 53–67. 10.1016/j.visres.2016.09.010 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shafritz K. M., Gore J. C., Marois R. (2002). The role of the parietal cortex in visual feature binding. Proc. Natl. Acad. Sci. U.S.A. 99 10917–10922. 10.1073/pnas.152694799 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shallice T. (2002). “ Fractionation of the supervisory system ,” in Principles of frontal lobe function , eds Stuss D. T., Knight R. T. (Oxford: Oxford University Press; ), 261–277. 10.1093/acprof:oso/9780195134971.003.0017 [ CrossRef ] [ Google Scholar ]
- Siegel J. M. (2001). The REM sleep-memory consolidation hypothesis. Science 294 1058–1063. 10.1126/science.1063049 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Smith E. E., Jonides J., Marshuetz C., Koeppe R. A. (1998). Components of verbal working memory: evidence from neuroimaging. Proc. Natl. Acad. Sci. U.S.A. 95 876–882. 10.1073/pnas.95.3.876 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sobczak J. (2017). Mechanisms of memory consolidation (dissertation). PhD thesis. York: University of York. [ Google Scholar ]
- Sorensen K. E. (2009). The connections of the hippocampal region new observations on efferent connections in the guinea pig, and their functional implications*. Acta Neurol. Scand. 72 550–560. 10.1111/j.1600-0404.1985.tb00914.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Speranza L., di Porzio U., Viggiano D., de Donato A., Volpicelli F. (2021). Dopamine: the neuromodulator of long-term synaptic plasticity, reward and movement control. Cells 10 : 735 . 10.3390/cells10040735 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Squire L. R., Wixted J. T. (2011). The cognitive neuroscience of human memory since H.M. Annu. Rev. Neurosci. 34 259–288. 10.1146/annurev-neuro-061010-113720 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Squire L. R., Zola S. M. (1996). Structure and function of declarative and nondeclarative memory systems. Proc. Natl. Acad. Sci. U.S.A. 93 13515–13522. 10.1073/pnas.93.24.13515 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Squire L. R., Genzel L., Wixted J. T., Morris R. G. (2015). Memory consolidation. Cold Spring Harb. Perspect. Biol. 7 : a021766 . 10.1101/cshperspect.a021766 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Squire L. R., Stark C. E. L., Clark R. E. (2004). The medial temporal lobe. Annu. Rev. Neurosci. 27 279–306. 10.1146/annurev.neuro.27.070203.144130 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sreenivasan K. K., Curtis C. E., D’Esposito M. (2014). Revisiting the role of persistent neural activity during working memory. Trends Cogn. Sci. 18 82–89. 10.1016/j.tics.2013.12.001 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stickgold R. (2005). Sleep-dependent memory consolidation. Nature 437 1272–1278. 10.1038/nature04286 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Swanson R. A., Levenstein D., McClain K., Tingley D., Buzsáki G. (2020). Variable specificity of memory trace reactivation during hippocampal sharp wave ripples. Curr. Opin. Behav. Sci. 32 126–135. 10.1016/j.cobeha.2020.02.008 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Takehara-Nishiuchi K. (2020). Prefrontal–hippocampal interaction during the encoding of New Memories. Brain Neurosci. Adv. 4 239821282092558 . 10.1177/2398212820925580 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Takeuchi T., Duszkiewicz A. J., Morris R. G. (2014). The synaptic plasticity and memory hypothesis: encoding, storage and persistence. Philos. Trans. R. Soc. B Biol. Sci. 369 : 20130288 . 10.1098/rstb.2013.0288 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tamminen J., Payne J. D., Stickgold R., Wamsley E. J., Gaskell M. G. (2010). Sleep spindle activity is associated with the integration of new memories and existing knowledge. The Journal of Neuroscience 30 14356–14360. 10.1523/jneurosci.3028-10.2010 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Thomas Yeo B. T., Krienen F. M., Sepulcre J., Sabuncu M. R., Lashkari D., Hollinshead M., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106 1125–1165. 10.1152/jn.00338.2011 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Thompson R. F., Kim J. J. (1996). Memory systems in the brain and localization of a memory. Proc. Natl. Acad. Sci. U.S.A. 93 13438–13444. 10.1073/pnas.93.24.13438 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Thuault S. J., Malleret G., Constantinople C. M., Nicholls R., Chen I., Zhu J., et al. (2013). Prefrontal cortex HCN1 channels enable intrinsic persistent neural firing and executive memory function. J. Neurosci. 33 13583–13599. 10.1523/jneurosci.2427-12.2013 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Todd J. J., Marois R. (2005). Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cogn. Affect. Behav. Neurosci. 5 144–155. 10.3758/cabn.5.2.144 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tononi G., Cirelli C. (2003). Sleep and synaptic homeostasis: a hypothesis. Brain Res. Bull. 62 143–150. 10.1016/j.brainresbull.2003.09.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tononi G., Cirelli C. (2014). Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81 12–34. 10.1016/j.neuron.2013.12.025 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tulving E. (1985). Memory and consciousness. Can. Psychol. 26 1–12. 10.1037/h0080017 [ CrossRef ] [ Google Scholar ]
- van Kesteren M. T. R., Ruiter D. J., Fernández G., Henson R. N. (2012). How schema and novelty augment memory formation. Trends Neurosci. 35 211–219. 10.1016/j.tins.2012.02.001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- van Kesteren M. T., Brown T. I., Wagner A. D. (2016). Interactions between memory and new learning: insights from fMRI multivoxel pattern analysis. Front. Syst. Neurosci. 10 : 46 . 10.3389/fnsys.2016.00046 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vilberg K. L., Rugg M. D. (2008). Memory retrieval and the PARIETAL CORTEX: a review of evidence from a dual-process perspective. Neuropsychologia 46 1787–1799. 10.1016/j.neuropsychologia.2008.01.004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vishnoi S., Naseem M., Raisuddin S., Parvez S. (2018). Behavioral tagging: plausible involvement of PKMZ, ARC and role of neurotransmitter receptor systems. Neurosci. Biobehav. Rev. 94 210–218. 10.1016/j.neubiorev.2018.07.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang J. H., Wang D., Gao Z., Chen N., Lei Z., Cui S., et al. (2016). Both glutamatergic and Gabaergic neurons are recruited to be associative memory cells. Biophys. J. 110 : 481a . 10.1016/j.bpj.2015.11.2571 [ CrossRef ] [ Google Scholar ]
- Wang J.-H., Cui S. (2018). Associative memory cells and their working principle in the brain. F1000Research 7 : 108 . 10.12688/f1000research.13665.1 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wig G. S., Buckner R. L., Schacter D. L. (2009). Repetition priming influences distinct brain systems: evidence from task-evoked data and resting-state correlations. J. Neurophysiol. 101 2632–2648. 10.1152/jn.91213.2008 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Williams J. N. (2020). The neuroscience of implicit learning. Language Learning 70 255–307. 10.1111/lang.12405 [ CrossRef ] [ Google Scholar ]
- Wiltgen B. J., Zhou M., Cai Y., Balaji J., Karlsson M. G., Parivash S. N., et al. (2010). The hippocampus plays a selective role in the retrieval of detailed contextual memories. Curr. Biol. 20 1336–1344. 10.1016/j.cub.2010.06.068 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Winocur G., Moscovitch M. (2011). Memory transformation and systems consolidation. J. Int. Neuropsychol. Soc. 17 766–780. 10.1017/S1355617711000683 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wolf R. C., Vasic N., Walter H. (2006). Differential activation of ventrolateral prefrontal cortex during working memory retrieval. Neuropsychologia 44 2558–2563. 10.1016/j.neuropsychologia.2006.05.015 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Xu Y., Chun M. M. (2006). Dissociable neural mechanisms supporting visual short-term memory for objects. Nature 440 91–95. 10.1038/nature04262 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Xu Y., Zou P., Cohen A. E. (2017). Voltage imaging with genetically encoded indicators. Curr. Opin. Chem. Biol. 39 1–10. 10.1016/j.cbpa.2017.04.005 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yang P., Wang M., Luo C., Ni X., Li L. (2022). Dissociable causal roles of the frontal and parietal cortices in the effect of object location on object identity detection: a TMS study. Exp. Brain Res. 240 1445–1457. 10.1007/s00221-022-06344-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yu R., Han B., Wu X., Wei G., Zhang J., Ding M., et al. (2023). Dual-functional network regulation underlies the Central Executive System in working memory. Neuroscience 524 158–180. 10.1016/j.neuroscience.2023.05.025 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yu S., Rempel S., Gholamipourbarogh N., Beste C. (2022). A ventral stream-prefrontal cortex processing cascade enables working memory gating dynamics. Commun. Biol. 5 : 1086 . 10.1038/s42003-022-04048-7 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zeithamova D., Preston A. R. (2010). Flexible memories: differential roles for medial temporal lobe and prefrontal cortex in cross-episode binding. J. Neurosci. 30 14676–14684. 10.1523/jneurosci.3250-10.2010 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhu Y., Zeng Y., Ren J., Zhang L., Chen C., Fernandez G., et al. (2022). Emotional learning retroactively promotes memory integration through rapid neural reactivation and reorganization. eLife 11 : e60190 . 10.7554/elife.60190 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zielinski M. C., Tang W., Jadhav S. P. (2018). The role of replay and Theta sequences in mediating hippocampal-prefrontal interactions for memory and cognition. Hippocampus 30 60–72. 10.1002/hipo.22821 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zlotnik G., Vansintjan A. (2019). Memory: an extended definition. Front. Psychol. 10 : 2523 . 10.3389/fpsyg.2019.02523 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Finally, we provide suggestions for future research to establish whether muscle memory exists in humans. 2. ... without any underlying loss of myonuclei as was also rightfully addressed by the authors of the original paper in their recent letter‐to‐the‐editor 13 as a response to this "secondary analysis." Based on this, as well as the ...
The concept of "muscle memory by myonuclear permanence" has mainly been based on data attained from rodent experimental models. Whether the postulated mechanism also holds true in humans remains largely ambiguous. Nevertheless, there are several studies in humans that provide evidence to potentially support or contradict (parts of) the muscle ...
To provide additional insight on the potential presence of muscle memory by myonuclear permanence in vivo in humans, we have re-analysed some of our previously performed exercise training studies. Finally, we provide suggestions for future research to establish whether muscle memory exists in humans. 2 CURRENT EVIDENCE FROM ANIMAL STUDIES
Skeletal muscle memory is an exciting phenomenon gaining significant traction across several scientific communities, among exercise practitioners, and the public. Research has demonstrated that skeletal muscle tissue can be "primed" by earlier positive encounters with exercise training that can enhance adaptation to later retraining, even following significant periods of exercise cessation ...
The term cell memory has also been used to describe irreversible programming of cells such as stem cells, and is attributed to epigenetic mechanisms such as histone and DNA modifications other than changes in base sequence (e.g. Alvarez and Margulies, 2014; Li and Zhang, 2014).For example, it has been demonstrated that fibroblasts in culture maintain a specific gene expression pattern ...
Skeletal muscle memory is an exciting phenomenon gaining significant traction across several scientific communities, among exercise practitioners, and the public. Research has demonstrated that skeletal muscle tissue can be "primed" by earlier positive encounters with exercise training that can enhance adaptation to later retraining, even ...
Skeletal muscle may possess a long-term DNA hypomethylation 'memory' of prior exercise training that could have consequences for future myofibres adaptability during retraining. 94-96 Future studies should evaluate the role of epigenetic 'memory' association with a first training period to extend our understanding of the molecular bases ...
The concept of "muscle memory by myonuclear permanence" has mainly been based on data attained from rodent experimental models. Whether the postulated mechanism also holds true in humans remains largely ambiguous. Nevertheless, there are several studies in humans that provide evidence to potentially support or contradict (parts of) the ...
In vivo time-lapse microscopy reveals no loss of murine myonuclei during weeks of muscle atrophy. J Clin Invest 118: 1450-1457, 2008. doi:10.1172/JCI34022. Bruusgaard JC, Johansen IB, Egner IM, Rana ZA, Gundersen K. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining.
Memory is a process in which information is encoded, stored, and retrieved. For vertebrates, the modern view has been that it occurs only in the brain. This review describes a cellular memory in skeletal muscle in which hypertrophy is 'remembered' such that a fibre that has previously been large, but subsequently lost its mass, can regain mass ...
It is unknown if adult human skeletal muscle has an epigenetic memory of earlier encounters with growth. We report, for the first time in humans, genome-wide DNA methylation (850,000 CpGs) and ...
Key points: Referring to the muscle memory theory, previously trained muscles acquire strength and volume much faster than naive muscles. Using extreme experimental models such as synergist ablation or steroid administration, previous studies have demonstrated that the number of nuclei increases when a muscle becomes enlarged, which serves as a cellular muscle memory mechanism for the muscle.
The article by Liu et al. (2011) reports that there are all-or-none action potentials in C. elegans muscles. This study from Zhao-Wen Wang's laboratory demonstrates that these action potentials are calcium dependent and occur in spontaneous trains. By recording from mutant animals, Liu and colleagues identified the ion channels that contribute ...
The Journal of Physiology publishes research in all areas of physiology and pathophysiology that illustrates new physiological principles or mechanisms. Key points Referring to the muscle memory theory, previously trained muscles acquire strength and volume much faster than naive muscles. ... Research Paper.
Due to the small and variable effects of the training on relevant parameters, we were unable to draw any conclusions one way or the other related to muscle memory in humans. We have previously suggested that myonuclei acquired from satellite cells during hypertrophy, and subsequently not lost, could serve as a mechanism for muscle memory ( 3, 6 ...
Muscle memory. In the movie 'Burn after reading', George Clooney sings the praises of 'muscle memory'- the concept that muscles have excitable circuitry capable of directing complex behaviour on their own. Sure enough, when the moment comes his muscles remember to rapidly draw a gun and shoot a man who inadvertently surprises him.
New evidence indicates that during hypertrophy, pre-existing muscle fibres recruit nuclei from satellite cells, which are not lost during atrophy, and the new permanent myonuclei represent cellular memory facilitating subsequent growth. ABSTRACT Memory is a process in which information is encoded, stored, and retrieved. For vertebrates, the modern view has been that it occurs only in the brain.
The memory residing in the muscle cells has all the classical characteristics of "memory" with encoding, storage and retrieval (Fig. 1 ). It can be encoded by de novo exercise, stored as an increased number of nuclei and retrieved by new training. Figure 1. Schematic illustration of a muscle memory demonstrating the encoding, storage and ...
Engaging in physical activity (exercise) can improve physical fitness by increasing muscle strength 1,2,3,4, bone density 5,6,7, cardiovascular performance 8,9, lung capacity 10 (although see 11 ...
A study led by researchers at Keele University has shown for the first time that human muscles possess a 'memory' of earlier growth -- at the DNA level. Periods of skeletal muscle growth are ...
I "Muscle memory" is a term commonly used in everyday discourse for the sort of embodied implicit memory that unconsciously helps us perform various motor tasks we have somehow learned through habituation, either through explicit, intentional training or simply as the result of informal, unintentional, or even unconscious learning from repeated prior experience.
Stanford neuroscientists observe memory formation in real time. Watch on. In their new study, published July 8, 2022 in Neuron, the researchers trained mice to use their paws to reach food pellets through a small slot. Using genetic wizardry developed by the lab of Liqun Luo, a Wu Tsai Neurosciences Institute colleague in the Department of ...
Working memory. Working memory is primarily associated with the prefrontal and posterior parietal cortex (Sarnthein et al., 1998; Todd and Marois, 2005).Working memory is not localized to a single brain region, and research suggests that it is an emergent property arising from functional interactions between the prefrontal cortex (PFC) and the rest of the brain (D'Esposito, 2007).