Department of Defense Congressionally Directed Medical Research Programs
Dod search tools & resources, research programs.
Breast Cancer Research Program (March Pre-app deadlines)
Peer Reviewed Medical Research Program (March/April Pre-app deadlines)
Amyotrophic Lateral Sclerosis Research Program (April Pre-app deadlines)
Ovarian Cancer Research Program (April/May Pre-app deadlines)
Autism Research Program (April Pre-app deadlines)
Epilepsy Research Program (May Pre-app deadlines)
Lung Cancer Research Program (May/July Pre-app deadlines)
Peer Reviewed Alzheimer's Research Program (May Pre-app deadlines)
Spinal Cord Injury Research Program (May Pre-app deadlines)
Tick-Borne Disease Research Program (May Pre-app deadlines)
Military Burn Research Program (May Pre-app deadlines)
Peer Reviewed Cancer Research Program (May/July Pre-app deadlines)
Tuberous Sclerosis Complex Research Program (June Pre-app deadlines)
Orthotics and Prosthetics Outcomes Research Program (June Pre-app deadlines)
Traumatic Brain Injury and Psychological Health Research Program (June Pre-app deadlines)
Joint Warfighter Medical Research Program (June Pre-app deadline)
Peer Reviewed Orthopaedic Research Program (June Pre-app deadlines)
Bone Marrow Failure Research Program (June Pre-app deadlines)
Rare Cancers Research Program (June/July Pre-app deadlines)
Multiple Sclerosis Research Program (June/July Pre-app deadlines)
Prostate Cancer Research Program (June/August Pre-app deadlines)
Lupus Research Program (July Pre-app deadlines)
Parkinson's Research Program (July Pre-app deadlines)
Reconstructive Transplant Research Program (July Pre-app deadlines)
Pancreatic Cancer Research Program (July/September Pre-app deadlines)
Combat Readiness-Medical Research Program (July Pre-app deadlines)
Vision Research Program (July/August Pre-app deadlines)
Melanoma Research Program (July/August Pre-app deadlines)
Kidney Cancer Research Program (July/September Pre-app deadlines)
Hearing Restoration Research Program (August Pre-app deadline)
Neurofibromatosis Research Program (August Pre-app deadlines)
Duchenne Muscular Dystrophy Research Program (August Pre-app deadlines)
Combat Casualty Care Research Program (August Pre-app deadline)
Chronic Pain Management Research Program (August Pre-app deadline)
Toxic Exposures Research Program (September Pre-app deadlines)
More programs to be posted soon. The most up to date information may be found on the DoD Funding Opportunities webpage.

U.S. Government Accountability Office

Biomedical Research: Observations on DOD's Management of Congressionally Directed Medical Research Programs
DOD awards funds to biomedical researchers for projects on topics identified by Congress. The projects contribute to the development of new drugs, vaccines, and medical devices.
DOD uses a cyclical, routine process to prioritize investments from these funds. In FYs 2015-19, it distributed nearly 100% of its $4.46 billion in funding.
DOD coordinates with the Department of Veterans Affairs and the National Institutes of Health, which also sponsor research. While we found a few DOD projects were similar in topic and methods to VA and NIH projects, their use of a shared database has helped identify and prevent overlap and duplication.

What GAO Found
The Department of Defense (DOD) awards funds from Congressionally Directed Medical Research Programs (CDMRP) appropriations to researchers for projects that focus on advancements in military medicine and public health benefits for areas such as cancers and substance abuse. A typical award ranges from less than $100,000 for a project with a new focus to millions of dollars for a clinical trial. Based on DOD data, for fiscal years 2015 through 2019, DOD obligated—committed government funds—nearly 100 percent of approximately $4.46 billion in available CDMRP appropriations. About 1.3 percent ($59.252 million) was unobligated for the period.
DOD uses a cyclical, routine process to prioritize and assess investments from CDMRP appropriations (see figure).
Department of Defense Cyclical, Routine Process for Prioritizing and Assessing CDMRP Investments
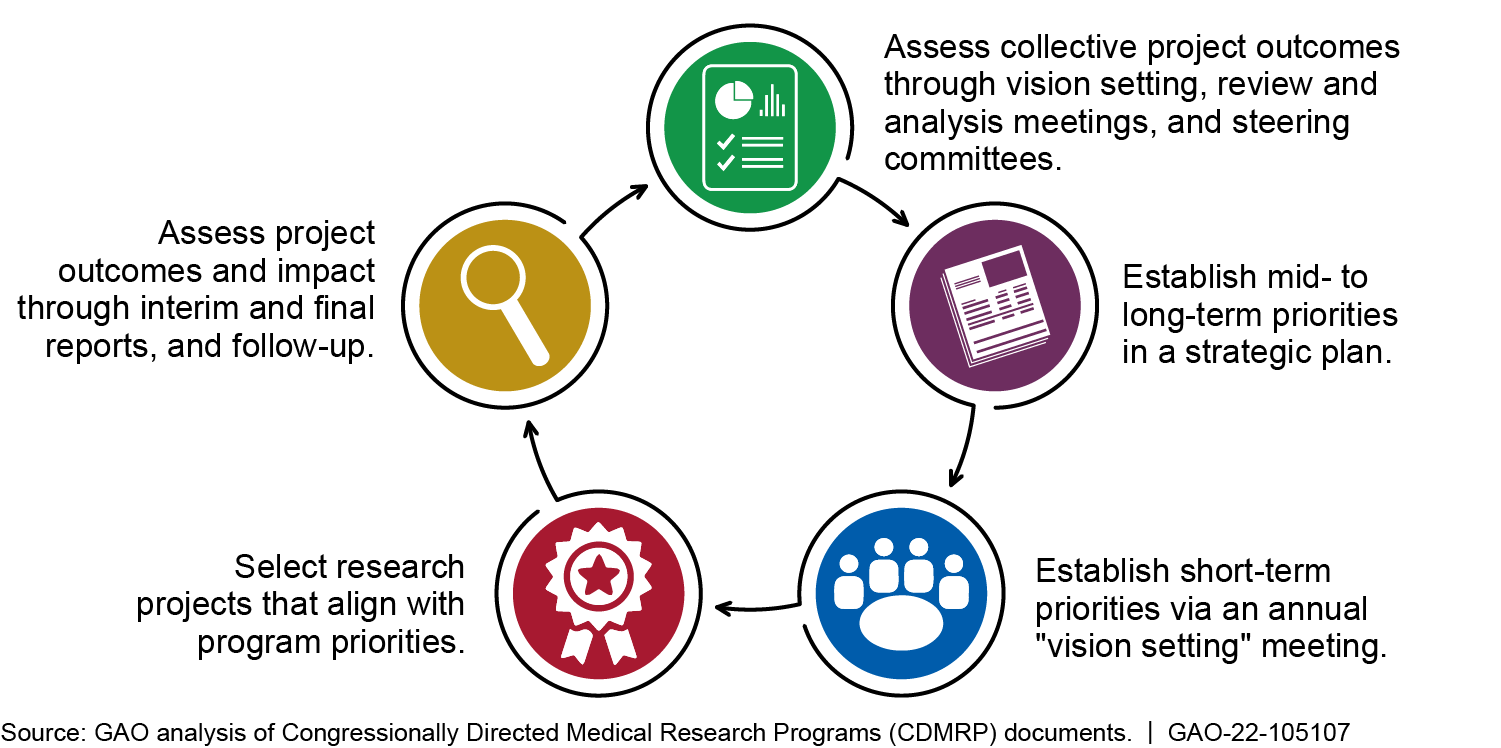
For example, each year following receipt of appropriations, DOD convenes a panel of experts for each program to help CDMRP staff agree on a strategy and priorities for the mid- and long-term and the upcoming year. These panels also review research applications and recommend ones to award funds based on their alignment with program priorities and expected impact, according to DOD officials. GAO's analysis of documents for 25 selected projects and the respective research programs found that each applicant clearly stated the relationship between the project and one or more program priorities.
The National Institutes of Health (NIH) and the Department of Veterans Affairs (VA) are significant federal sponsors of biomedical research. DOD coordinates with NIH and VA for CDMRP planning and project selection throughout the process shown in the above figure. For program planning during annual "vision setting," panels discuss research sponsored by other organizations and help ensure CDMRP investments are complementary. The panels generally include officials from NIH, VA, or both.
CDMRP also coordinates with NIH and VA by leveraging shared data to identify and mitigate project overlap. In response to a 2012 GAO report, DOD and NIH implemented an electronic interface between their research administration systems through the NIH Query View Report system, which includes VA data. CDMRP's full implementation of the system began in fiscal year 2019, when staff used it during application reviews to identify potential overlap with NIH and VA research. According to DOD officials, using shared data has helped them identify overlap and duplication in research projects, which led them to take mitigation steps and avoid cost for the federal government. In comparing 25 selected CDMRP projects with NIH- and VA- projects funded during the same period, GAO found that some projects were similar in topic and methods. GAO identified two projects (one from CDMRP and one from NIH) that contained verifiable overlap, which program managers had identified and addressed. In this instance, CDMRP and the researcher reduced the project's scope and budget. Through these and other coordination activities that are consistent with leading practices for collaboration, CDMRP is able to avoid and mitigate overlap and duplication of biomedical research efforts across DOD, NIH, and VA.
Why GAO Did This Study
DOD is among the United States' largest federal sponsors of biomedical research. For fiscal year 2021, DOD's appropriations included about $1.5 billion for 36 research programs known collectively as CDMRP. This represents a significant increase from CDMRP's initial appropriation in 1992 of $210 million for a breast cancer program.
The Joint Explanatory Statement accompanying the Consolidated Appropriations Act, 2021 includes a provision for GAO to review DOD’s CDMRP. This report provides information on CDMRP’s (1) execution of annual appropriations; (2) efforts to prioritize and assess biomedical research programs and investments; and (3) coordination of biomedical research with NIH and VA.
To address these objectives, GAO:
- analyzed DOD budget data for each CDMRP program for fiscal years 2015 through 2019 appropriation years;
- reviewed planning documents and research outcomes for a nongeneralizable sample of five programs for fiscal years 2018 through 2020 selected on the basis of size, subject, and research organizations awarded;
- analyzed records for 25 projects selected randomly from each selected program;
- reviewed reports and memorandums on research partnerships and collaboration;
- compared CDMRP research abstracts for selected projects with abstracts for NIH- and VA-funded projects to identify similarities and understand coordination steps; and
- interviewed officials from DOD, NIH, and VA.
For more information, contact Elizabeth Field at (202) 512-2775 or [email protected] .
Full Report
Gao contacts.
Elizabeth Field Director [email protected] (202) 512-4300
Office of Public Affairs
Sarah Kaczmarek Acting Managing Director [email protected] (202) 512-4800
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.

Evaluation of the Congressionally Directed Medical Research Programs Review Process
National Academies of Sciences, Engineering, and Medicine ; Health and Medicine Division ; Board on the Health of Select Populations ; Committee on the Evaluation of Research Management by DoD Congressionally Directed Medical Research Programs (CDMRP) .
- Copyright and Permissions
The medical research landscape in the United States is supported by a variety of organizations that spend billions of dollars in government and private funds each year to seek answers to complex medical and public health problems. The largest government funder is the National Institutes of Health (NIH), followed by the Department of Defense (DoD). Almost half of DoD's medical research funding is administered by the Congressionally Directed Medical Research Programs (CDMRP).
The mission of CDMRP is to foster innovative approaches to medical research in response to the needs of its stakeholders—the U.S. military, their families, the American public, and Congress. CDMRP funds medical research to be performed by other government and nongovernmental organizations, but it does not conduct research itself. The major focus of CDMRP funded research is the improved prevention, diagnosis, and treatment of diseases, injuries, or conditions that affect service members and their families, and the general public. The hallmarks of CDMRP include reviewing applications for research funding using a two-tiered review process, and involving consumers throughout the process. Evaluation of the Congressionally Directed Medical Research Programs Review Process evaluates the CDMRP two-tiered peer review process, its coordination of research priorities with NIH and the Department of Veterans Affairs, and provides recommendations on how the process for reviewing and selecting studies can be improved.
- Collapse All
- The National Academies of SCIENCES • ENGINEERING • MEDICINE
- COMMITTEE ON THE EVALUATION OF RESEARCH MANAGEMENT BY DOD CONGRESSIONALLY DIRECTED MEDICAL RESEARCH PROGRAMS (CDMRP)
- Study Staff
- Acknowledgments
- Acronyms and Abbreviations
- COMMITTEE'S STATEMENT OF TASK AND APPROACH
- THE CDMRP REVIEW PROCESS
- PARTICIPANTS IN THE PROCESS
- CONSUMER ENGAGEMENT
- FINDINGS AND RECOMMENDATIONS
- CONCLUSIONS
- PRIOR REPORTS ON CDMRP
- CHARGE TO THE 2016 COMMITTEE
- COMMITTEE'S APPROACH TO ITS CHARGE
- ORGANIZATION OF THE REPORT
- THE CURRENT CDMRP
- CDMRP FUNDING
- OVERVIEW OF THE CDMRP REVIEW PROCESS
- CDMRP PERSONNEL
- CDMRP CONTRACTORS
- REVIEW PANELS
- STAKEHOLDERS MEETINGS
- THE VISION SETTING PROCESS
- PRE-APPLICATION RECEIPT AND SCREENING
- PRE-MEETING ACTIVITIES
- THE PEER REVIEW MEETING
- POST-MEETING ACTIVITIES AND DELIVERABLES
- THE PROGRAMMATIC REVIEW MEETING
- MEDICAL RESEARCH FUNDING IN THE UNITED STATES
- COMMITTEE'S APPROACH TO COORDINATION
- ESTABLISHING RESEARCH PRIORITIES
- AVOIDING RESEARCH DUPLICATION
- STRATEGIC PLANNING
- COORDINATION
- TRANSPARENCY
- STANDARDIZATION
- IMPLEMENTION OF THE 1993 AND 1997 IOM RECOMMENDATIONS
- Appendix A. Committee Biographical Sketches
- Appendix B. Open Session Agendas
Suggested citation:
National Academies of Sciences, Engineering, and Medicine. 2016. Evaluation of the Congressionally Directed Medical Research Programs Review Process. Washington, DC: The National Academies Press. doi: 10.17226/23652.
- Cite this Page National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on the Health of Select Populations; Committee on the Evaluation of Research Management by DoD Congressionally Directed Medical Research Programs (CDMRP). Evaluation of the Congressionally Directed Medical Research Programs Review Process. Washington (DC): National Academies Press (US); 2016 Dec 19. doi: 10.17226/23652
- PDF version of this title (3.3M)
Related information
- NLM Catalog Related NLM Catalog Entries
Similar articles in PubMed
- Developing a Research Strategy for Suicide Prevention in the Department of Defense: Status of Current Research, Prioritizing Areas of Need, and Recommendations for Moving Forward. [Rand Health Q. 2014] Developing a Research Strategy for Suicide Prevention in the Department of Defense: Status of Current Research, Prioritizing Areas of Need, and Recommendations for Moving Forward. Ramchand R, Eberhart NK, Guo C, Pedersen ER, Savitsky TD, Tanielian T, Voorhies P. Rand Health Q. 2014 Dec 30; 4(3):16. Epub 2014 Dec 30.
- Enhancing Health Care in the Veteran Community Through Synergistic Research Funding. [Front Psychiatry. 2021] Enhancing Health Care in the Veteran Community Through Synergistic Research Funding. Lane MD, Lidie KB, Santullo RF, Dalal SJ. Front Psychiatry. 2021; 12:541889. Epub 2021 Feb 18.
- Utilization of the Department of Defense Peer-Reviewed Orthopaedic Research Program (PRORP): Combating Musculoskeletal Disease With PRORP. [J Am Acad Orthop Surg. 2022] Utilization of the Department of Defense Peer-Reviewed Orthopaedic Research Program (PRORP): Combating Musculoskeletal Disease With PRORP. Anderson AB, Grazal CF, Tintle SM, Potter BK, Forsberg JA, Dickens JF. J Am Acad Orthop Surg. 2022 Mar 1; 30(5):195-205.
- Funding priorities in animal reproduction at the United States Department of Agriculture's Cooperative State Research, Education, and Extension Service. [Biol Reprod. 2006] Funding priorities in animal reproduction at the United States Department of Agriculture's Cooperative State Research, Education, and Extension Service. Mirando MA, Hamernik DL. Biol Reprod. 2006 Mar; 74(3):459-62. Epub 2005 Nov 16.
- Review Acute Pathophysiology of Blast Injury—From Biomechanics to Experiments and Computations: Implications on Head and Polytrauma. [Brain Neurotrauma: Molecular, ...] Review Acute Pathophysiology of Blast Injury—From Biomechanics to Experiments and Computations: Implications on Head and Polytrauma. Chandra N, Sundaramurthy A. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. 2015
Recent Activity
- Evaluation of the Congressionally Directed Medical Research Programs Review Proc... Evaluation of the Congressionally Directed Medical Research Programs Review Process
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Official websites use .gov
Secure .gov websites use HTTPS

Department of Defense Awards $46.8 Million in Grants for Minerva Research Initiative
By Toni DeVille
The Department of Defense today announced $46.8 million in grants to 19 university-based faculty teams under its Minerva Research Initiative. These three- to five-year awards support basic research in social and behavioral sciences on topics relevant to U.S. national security.
To read the full announcement, click here .

working collectively to protect and enrich the congressionally directed medical research programs under the department of defense .

Defense Health Research Consortium to Congress: Enact the FY24 Defense Appropriations Act
November 9, 2023
131 Patient Advocacy Organizations Call for Full Funding for Congressionally Directed Medical Research Programs (CDMRP)
The Defense Health Research Consortium and dozens of its affiliated members sent a letter to House and Senate leadership , calling on them to “work toward the enactment of the fiscal year 2024 Defense Appropriations Act, to ensure full funding levels for the Defense Health Research Programs, including the Congressionally Directed Medical Research Programs (CDMRP).”
The letter, signed by 131 patient advocacy organizations, health and medical associations, veterans organizations and colleges and universities, expresses concerns “about the possibility of Congress enacting additional short-term continuing resolutions that would delay final consideration of the appropriations bills until next year.” The letter also expresses even more serious concerns about a government shutdown which “would completely halt all operations of CDMRP and be highly disruptive to plans for soliciting, reviewing and evaluating grant applications and administering existing awards.”
The Defense Health Research Consortium was founded by CRD Associates to bring a diverse community together to advocate for the CDMRP, a $1.5 billion medical research program designed to meet the unique health and medical challenges of the men and women in the U.S. Armed Services. Funding for the CDMRP is added every year by Congress to the annual Defense Appropriations Act, and this funding has faced threats and similar challenges in previous years.
For more information about how CRD Associates can help your organization make a difference through CDMRP, please reach out to Mark Vieth at [email protected] .
Defense Health Research Consortium to Congress: Enact the FY23 Defense Appropriations Bill
The Defense Health Research Consortium and dozens of its affiliated members today sent a letter to House and Senate leadership, calling on them to “work toward the enactment of the fiscal year 2023 Defense Appropriations Act, to ensure full funding levels for the Defense Health Research Programs, including the Congressionally Directed Medical Research Programs (CDMRP).” The letter, signed by 121 patient advocacy organizations, health and medical associations, veterans organizations and colleges and universities, warns of...
Senate Appropriations Committee Approves FY2024 Funding for CDMRP
July 28, 2023 Yesterday, by an overwhelming bipartisan vote of 27-1, the Senate Committee on Appropriations approved its version of the fiscal year 2024 Defense Appropriations Act. This legislation provides funding for the Congressionally Directed Medical Research Program (CDMRP) consistent with levels provided in previous years by the Committee. Specifically, the bill provides funding for a number of cancer programs, including breast cancer ($130 million), prostate cancer ($75 million), ovarian cancer ($15 million), and rare...
LATEST NEWS
June 26, 2023
House Appropriations Committee approves FY24 Defense Appropriations Act
On June 22, the House Committee on Appropriations approved its version of the fiscal year 2024 Defense Appropriations Act. Approved 34 to 24 along party lines, the bill would provide $826.45 billion for defense spending in the fiscal year starting October 1, staying within spending caps negotiated in the debt ceiling agreement.
The House Committee mark largely includes funding for the Congressionally Directed Medical Research Programs (CDMRPs) at existing fiscal year 2023 levels. The bill provides $10 million to create a new arthritis research program. Arthritis is currently an eligible condition in the Peer Reviewed Medical Research Program (PRMRP).
The House version of the bill will likely be brought to the House floor for consideration in July. The House may choose to first consider the fiscal year 2024 National Defense Authorization Act when it reconvenes on July 11.
In a related development, on June 22, the Senate Committee on Appropriations approved allocations for its versions of the fiscal year 2024 appropriations bills, including $823.3 billion for defense spending.
_______________________
November 22, 2022
The Defense Health Research Consortium and dozens of its affiliated members today sent a letter to House and Senate leadership, calling on them to “work toward the enactment of the fiscal year 2023 Defense Appropriations Act, to ensure full funding levels for the Defense Health Research Programs, including the Congressionally Directed Medical Research Programs (CDMRP).”
November 16, 2022
Rep. Ken Calvert has survived his re-election and will serve as chair of HAC-D
End-of-Year Outlook: What’s Left on the Congressional Agenda
Congress returns to Washington next week with a full agenda before adjourning for the year. Here is a look at what issues they may consider:
FY 2023 Appropriations
One benefit of the close elections is the path to finishing fiscal year (FY) 2023 appropriations may have gotten easier as Republicans will not have the leverage to punt the spending package into the new Congress. The current CR expires on December 16 th and Democrats in the House and Senate intend to negotiate and enact an omnibus spending package by then, or by the end of the calendar year. An omnibus would likely include supplemental Ukraine funding, disaster relief, mental health authorizations, and other priorities.
The $31.4 trillion debt limit will need to be raised by before the end of 2023. Influential Republicans have described the debt limit as a tool they will use to extract major spending cuts, despite the risk of crashing the economy. Therefore, Democrats and moderate Republicans are discussing the possibility of including a debt limit increase, or abolishing the debt limit, during the lame duck. President Biden opposes the abolishment of the debt limit, claiming it would be “irresponsible.” It is unclear there will be enough votes attached to this proposal to the omnibus. However, it would protect the economy under President Biden while solving the issue for the next Congress by taking it out of their hands.
Congress Passes Another Continuing Resolution to Extend Fiscal Deadline Through December 18
December 11, 2020
The Senate passed a one-week continuing resolution (CR) late Friday afternoon via voice vote, sending it to President Trump for his signature just hours before the midnight deadline. The President signed the bill Friday evening to keep the government open for another week while lawmakers work to reach an agreement on a spending package before the new December 18 deadline.
Lawmakers and staff worked over the weekend to finalize and file an omnibus package by COB today, but sources tell us lawmakers are still uncertain whether it is possible. Rumors are circulating that House Appropriations Committee staff have drafted a three-month CR that they could rely on if a final spending package isn’t ready by early this week. However, appropriators in both chambers are outwardly optimistic, including Senate Majority Leader Mitch McConnell (R-KY), who told reporters he is hopeful that progress on these items will produce a final bill this week.
Senate Passes FY 21 NDAA Conference Report
The Senate this afternoon passed H.R. 6395, the $740.5 billion FY 21 NDAA conference report, by a vote of 84 to 13.
President Trump had threatened to veto the bill because it excluded language repealing a legal shield to tech companies and included bill language that calls for renaming military bases named after confederate soldiers. The House passed the bill earlier this week by a strong vote of 335-78.
Congress Passes Continuing Resolution, Extending Fiscal Deadline
September 30, 2020
Today, Congress passed the continuing resolution (CR) to extend the fiscal deadline through December 11. According to a person familiar with the planning, President Trump will sign the CR on Thursday, but there won’t be a lapse in appropriations because of his intent to sign the measure, which the Senate cleared Wednesday on an 84-10 vote, several days after the House voted 359 to 57 to approve the bipartisan bill.
House Democrats Unveil a Short-term Spending Bill
September 22, 2020
House Appropriations Committee Chair Nita Lowey (D-NY) introduced a short-term CR to extend Fiscal Year (FY) 2020 funding beyond the September 30 fiscal deadline until December 11.
The Democrats reportedly introduced the bill on their own without support of the White House or House or Senate Republicans.
The Latest on the CR Negotiations
September 15, 2020
We are hearing the House is planning on filing a continuing resolution on Friday, and plan to take it up on the floor next week. How long the CR will last is still unknown, but it seems that Speaker Pelosi is in favor of February or March. Senate Majority Leader McConnell and Treasury Secretary Mnuchin unsurprising prefer the CR expire in December.
House Approves Fiscal Year 2021 Defense Appropriations Act
July 31, 2020
The House has approved the Fiscal Year 2021 Defense Appropriations Act, as part of a larger minibus package. There were no amendments that would have adversely impacted the CDMRPs. Now on to the Senate, which may not act on this until after the election.
The House has sent its members home for August but will call them back with a 24 hour notice if there is a deal on the next COVID-19 relief package.

EXTERNAL LINKS
- Congressionally Directed Medical Research Programs (CDMRP) – Home Page
- CDMRP – Research Programs Page
Official websites use .gov
Secure .gov websites use HTTPS
Department of Defense Awards $190 Million Cooperative Agreement to Deliver National STEM Education and Outreach Programs
The Department of Defense today announced the award of the Defense Science, Technology, Engineering, and Mathematics Education Consortium (DSEC) Cooperative Agreement to the Research Triangle Institute and its consortium of over 25 regional and national partners to provide STEM education and outreach programs in communities across the nation. These programs are foundational to the department's strategy to build a 21st century workforce with the skills and talent to meet evolving defense challenges.
The consortium will receive up to $190 million over the course of 10 years to implement a diversity of STEM programming, allowing the department to increase the permeability of ideas into its science and engineering workforce. These partnerships will deliver far‐reaching sustainable and scalable programs, providing unique hands-on learning experiences where students can work side-by-side with the nation's best scientists and engineers on cutting-edge research and development.
"Outreach is incredibly important for the Defense Department, and we continue to seek out the best and brightest minds across the nation," said Dr. Aprille Ericsson, assistant secretary of defense for science and technology. "Our men and women in uniform depend on talent and expertise within our science and technology workforce to out-innovate our peer adversaries, and I consider development, recruitment, and retention of STEM talent to be one of my top priorities."
DSEC, part of the National Defense Education Program (NDEP), is a vehicle for DoD to partner with like-minded organizations to identify and support effective activities for pre-K–12 students, educators, and parents, as well as activities that continue to engage students at the undergraduate level. Under this cooperative agreement, RTI will address five required DSEC Fundamental Elements with a qualified team of organizations, including American Institutes for Research, Left Bank Consulting, and Teaching Institute for Excellence in STEM. These five elements require the consortium to leverage partnerships to amplify awareness of opportunities and broaden impact, evolve programming based on data and evaluations, provide coherent and interconnected Pre-K–12 STEM education and outreach, and, where feasible, engage DoD scientists and engineers with students and educators.
Headquartered in Research Triangle Park, North Carolina, RTI will receive an initial award of up to $37 million over a two-year base period, with the possibility of four two-year option periods of up to $37.5 to $39 million per period. Awards to the remaining 20-plus partner organizations will be announced in the coming months.
About DoDSTEM
Department of Defense Science, Technology, Engineering, and Mathematics (DoDSTEM) comprises the Department's collective efforts in STEM talent development, including programs from the Departments of the Army, Navy, Air Force, other Defense Agencies, and NDEP. The DoDSTEM mission is to inspire, cultivate, and develop exceptional STEM talent through a continuum of opportunities across the Pre-K–20 grade levels, to enrich the nation's current and future DoD workforce poised to tackle evolving defense technological challenges. Learn more at https://dodstem.us/ .
Subscribe to Defense.gov Products
Choose which Defense.gov products you want delivered to your inbox.
Defense.gov
Helpful links.
- Live Events
- Today in DOD
- For the Media
- DOD Resources
- DOD Careers
- Help Center
- DOD / Military Websites
- Agency Financial Report
- Value of Service
- Taking Care of Our People
- FY 2025 Defense Budget
- National Defense Strategy
The Department of Defense provides the military forces needed to deter war and ensure our nation's security.
$28M Federal Grant to Fund Medical Innovations from Dartmouth Health Research
Dartmouth Health’s Dartmouth Hitchcock Medical Center (DHMC) received a $27.7 million, seven-year grant from the National Institutes of Health (NIH) to fund projects that will speed the implementation of proven medical innovations as part of a national consortium of biomedical research centers.
NIH’s Clinical and Translational Science Award (CTSA) will fund Dartmouth SYNERGY, also known as the Dartmouth Clinical and Translational Science Institute, a joint initiative that includes Dartmouth Health, Dartmouth College’s Geisel School of Medicine and Thayer School of Engineering; the White River Junction VA Medical Center, and collaborating institutions in Vermont and Maine. SYNERGY joins 60 other CTSAs in the United States and is one of eight with a significant focus on rural healthcare delivery.
“This award is an important milestone in the development of the biomedical research enterprise in the Upper Valley,” said Joanne M. Conroy, MD (D'77), CEO and president of Dartmouth Health. “The strength of SYNERGY is in its ability to bring together the resources of the health delivery system with the research and teaching assets of our partners at Dartmouth College, and regional collaborators at MaineHealth and the University of Vermont.”
With 50 percent of northern New England’s population living in rural areas, the challenges of rural healthcare delivery are a particular focus of SYNERGY’s work, Conroy said. “ Rural Americans are more likely to develop chronic illnesses and die compared to their urban counterparts. However, we are at an exciting time of breakthroughs and improvements in healthcare. This grant fortifies the infrastructure for SYNERGY to drive these innovations from the laboratories to the bedside and beyond.”
Translational science is a growing field that seeks to turn discoveries made in the laboratory, clinics and community settings into interventions that improve the health of individuals and populations. SYNERGY initially received a five-year CTSA grant in 2013. SYNERGY focuses on finding ways to overcome barriers to the adoption of ideas, tools, and treatment approaches that have been proven to work.
Steven L. Bernstein, MD, chief research officer at DHMC, Senior Associate Dean for Clinical and Translational Research and Professor of Emergency Medicine at Geisel, says a major element of SYNERGY’s successful grant proposal was embedding individuals with translational science expertise in operational roles in healthcare systems. Typically, researchers who have developed and proven the success of a treatment method or tool must reach out to administrators and providers and persuade them to consider new approaches. At DHMC, there are already clinicians and others with training in healthcare delivery science, implementation science, quality improvement, and related fields who are best positioned to facilitate the adoption of new evidence-based therapies, diagnostics, and practices.
“It was a strength for us – because we were already doing it,” Bernstein said. “What we are learning, we can generalize for others to learn from and adopt.”
SYNERGY will play a role in training more translational scientists. Part of this work will be led by Anna N.A. Tosteson, ScD, James J. Carroll 1948 Professor of Oncology in the Dartmouth Institute of Health Policy and Practice. Bernstein notes that Tosteson, one of three principal investigators on the CTSA, has already received another federal grant focusing on training in learning health system science that will complement the work of SYNERGY.
The development of technology to improve healthcare is another important element of SYNERGY. The third principal investigator in the CTSA is Keith D. Paulsen, PhD, the MacLean Professor of Engineering at Thayer and director of the Center for Surgical Innovation at DHMC. Paulsen is an expert on medical imaging technologies that have improved the accuracy and safety of complex surgical procedures.
Share this:
Search news, upcoming events.
- Special DCC Grand Rounds with Andrew Trister, MD, PhD August 29, 2024
- Department of Medical Education Faculty Development September 3, 2024 "Teaching scientific information to medical students should incorporate videos and abadon in-class attendance: discuss." September 3, 2024
- Pediatric Grand Rounds September 4, 2024
- Implementation Science Fundamentals Seminar September 4, 2024
- Thesis Defense (Taewook Kang) September 5, 2024
- M&I Graduate Seminar September 5, 2024
- Pediatric Grand Rounds September 11, 2024
- 2024 Academic Advancement, Promotion and Tenure Guidelines September 11, 2024
- Discovery Science Seminar Series - Wade Harper, PhD September 16, 2024
- Pediatric Grand Rounds September 18, 2024
- Psychiatry Grand Rounds September 19, 2024
- M&I - Second Annual Gary Heussler Alumni Lecture - Kiah Butler, Ph.D. September 20, 2024
Geisel in the News
Ai in medicine: a national registry could help increase transparency, experts say – stat news, safety net primary care capabilities after the covid-19 pandemic – jama network, valley parents column: the back-to-school paradox – valley news, clinical implications of new drinking water regulation for ‘forever chemicals’ – jama, unresponsive brain-damaged patients may have some awareness – the new york times.
This page may link to PDF files. Use this link to download Adobe Reader if needed.
Official websites use .gov
Secure .gov websites use HTTPS

U.S. Relations With Marshall Islands
Bilateral Relations Fact Sheet
Bureau of East Asian and Pacific Affairs
December 9, 2021
More information about the Marshall Islands is available on the Marshall Islands country page and from other Department of State publications and other sources listed at the end of this fact sheet.
U.S.-MARSHALL ISLANDS (RMI) RELATIONS
In 1947, the United Nations assigned the United States administering authority over the Trust Territory of the Pacific Islands (Trust Territory), which included the Republic of the Marshall Islands (RMI). The Compact of Free Association between the United States and the RMI entered into force in 1986. The Compact reflected that the RMI was a sovereign nation in free association with the United States. An Amended Compact entered into force in 2004. The Amended Compact does not have an end date.
The RMI is a sovereign nation. The United States and the RMI have full diplomatic relations and maintain deep ties and a cooperative relationship. The RMI government conducts its own foreign relations, consistent with the terms of the Amended Compact. Under the Amended Compact, the RMI and the United States agreed that the United States has full authority and responsibility for defense and security matters in and relating to the RMI. In addition, eligible RMI citizens can travel to the United States without visas to live, work, and study. RMI citizens also can serve in the U.S. Armed Forces and volunteer at per capita rates higher than many U.S. states. The RMI hosts the U.S. Army Garrison Kwajalein Atoll, including the Ronald Reagan Ballistic Missile Defense Test Site, a key component of the U.S. missile defense network.
The United States carried out 67 nuclear tests in what is now the northern Marshall Islands between 1946 and 1958. The Compact and the Section 177 Settlement Agreement, which entered into force in 1986, constitute the full settlement of all claims, past, present, and future, of the government, citizens and nationals of the Marshall Islands related to the Nuclear Testing Program. The United States has provided more than $600 million to the affected communities. Adjusting for inflation, this is more than $1 billion in current dollars. This includes direct financial settlement of nuclear claims, resettlement funds, rehabilitation of affected atolls, and radiation related health care costs.
U.S. Assistance to the Marshall Islands
Pursuant to the Amended Compact, the U.S. government provides economic and program assistance to the RMI. The United States provides more than $80 million in assistance every year, along with a variety of federal programs and services, until FY 2023, including contributions to a jointly managed trust fund. The assistance provisions are aimed to assist the RMI in its efforts to promote economic advancement and self-sufficiency. This assistance also includes grant assistance focused on six sectors: 1) education; 2) health; 3) infrastructure; 4) public sector capacity building; 5) private sector development; and 6) the environment. These grants are funded through and administered by the Department of the Interior. The Governments of the United States and the RMI established a Joint Economic Management and Financial Accountability Committee (JEMFAC), consisting of representatives of both nations, which is responsible for reviewing the audits and reports required under the Compact, evaluating the RMI’s progress in meeting development objectives and recommending ways to increase the effectiveness of U.S. Compact assistance, among other things.
A number of federal programs and services are provided pursuant to the Compact and relevant subsidiary agreements, including programs and services provided by the Federal Aviation Administration, the U.S. Postal Service, and others. In addition, reflecting the strong legacy of trusteeship cooperation, other U.S. federal agencies operate programs in the RMI consistent with U.S. laws, including the Department of Agriculture, Department of Health and Human Services, and Department of Education.
Separately, the United States provides foreign assistance to benefit RMI. For example, the RMI is highly vulnerable to natural disasters and the effects of climate change. U.S. foreign assistance also focuses on strengthening the RMI’s resilience to climate impacts.
Bilateral Economic Relations
The United States has a productive trade arrangement with the RMI under the Amended Compact. The RMI uses the U.S. dollar. The U.S. Army Garrison Kwajalein Atoll is the second-largest employer in the Marshall Islands. In 2020, the United States exported $120 million in goods to the Marshall Islands and imported $30.8 million in goods from the Marshall Islands.
The Marshall Islands is one of 16 Pacific Island countries which is part of the South Pacific Tuna Treaty with the United States. The treaty allows for U.S. purse seine vessels to fish in the exclusive economic zones of the Pacific Island parties. The treaty is viewed as a model of international and fishery cooperation and has helped establish fisheries observer and data reporting requirements, as well as monitoring, control, and surveillance standards for the region’s fisheries, all of which are vital to deterring illegal, unreported, and unregulated fishing.
RMI’s Membership in International and Regional Organizations
The Marshall Islands and the United States belong to a number of the same international organizations, including the United Nations, International Monetary Fund, World Bank, the International Atomic Energy Agency, and Asian Development Bank.
The Marshall Islands is a member of the Pacific Islands Forum, the Pacific Regional Environment Programme (SPREP), and the Pacific Community (SPC). The Marshall Islands also is a member of the Western and Central Pacific Fisheries Commission (WCPFC). In addition, the Marshall Islands is one of the eight signatories of the Nauru Agreement Concerning Cooperation in the Management of Fisheries of Common Interest that collectively harvests approximately 55 percent of the western and central Pacific tuna supply – a globally significant fishery that provides 30 percent of the world’s tuna supply.
Bilateral Representation
Principal embassy officials are listed in the Department’s Key Officers List .
The Marshall Islands maintains an embassy in the United States at 2433 Massachusetts Avenue NW, Washington, DC 20008 (tel. 202-234-5414). The RMI Ambassador to the United States is Gerald Zackios. The RMI maintains consulates in Springdale, Arkansas, and Honolulu, Hawaii.
More information about the Marshall Islands is available from the Department of State and other sources, some of which are listed here:
CIA World Factbook Marshall Islands Page U.S. Embassy U.S. Census Bureau Foreign Trade Statistics Travel Information U.S. Citizenship and Immigration Services
U.S. Department of State
The lessons of 1989: freedom and our future.

Clade I Mpox virus genomic diversity in the Democratic Republic of the Congo, 2018 - 2024: Predominance of Zoonotic Transmission
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Eddy Kinganda-Lusamaki
- For correspondence: [email protected] [email protected]
- ORCID record for Adrienne Amuri-Aziza
- ORCID record for Nicolas Fernandez
- ORCID record for Jean-Claude Makangara-Cigolo
- ORCID record for Catherine Pratt
- ORCID record for Emmanuel Hasivirwe Vakaniaki
- ORCID record for Nicole A. Hoff
- ORCID record for Sabin Sabiti Nundu
- ORCID record for Sydney Merritt
- ORCID record for Nicola Low
- ORCID record for Hugo Kavunga-Membo
- ORCID record for Laurens Liesenborghs
- ORCID record for Tony Wawina-Bokalanga
- ORCID record for Koen Vercauteren
- ORCID record for Daniel Mukadi-Bamuleka
- ORCID record for Lorenzo Subissi
- ORCID record for Jean-Jacques Muyembe
- ORCID record for Jason Kindrachuk
- ORCID record for Eric Delaporte
- ORCID record for Sofonias Tessema
- ORCID record for Eric D’Ortenzio
- ORCID record for Anne W. Rimoin
- ORCID record for Lisa E. Hensley
- ORCID record for Placide Mbala-Kingebeni
- ORCID record for Martine Peeters
- Info/History
- Supplementary material
- Preview PDF
Background Recent reports raise concerns on the changing epidemiology of mpox in the Democratic Republic of the Congo (DRC), with increasing case counts, sexual contact-mediated clusters, and sustained human-to-human transmission driven by a novel monkeypox virus (MPXV) subclade, clade Ib. However, only a limited number of clade I MPXV genomes have been characterized so far, from a limited number of regions.
Methods We conducted whole genome sequencing of 603 mpox-positive samples that were collected from 581 patients between 2018-2024 in 17 of the 26 provinces of the DRC.
Results Genome coverage was at least 70% for 429/603 (71.1%) samples and near full-length MPXV genomes (>90% coverage) were obtained for 348/603 (57.7%) samples from 337 patients. All newly generated MPXV sequences belonged to clade I, among which 17 were clade Ib strains, all from patients infected in 2024 in the South-Kivu province. The large majority (>95%) of the new strains fall within previously described clade Ia groups and potential new groups have also been observed. The low number of APOBEC3 mutations found among clade Ia suggests that most human mpox cases are probably linked to zoonotic transmissions. Genetically diverse MPXV lineages co-circulate in small geographic areas during the same outbreak suggesting multiple zoonotic introductions over a short period from one or multiple reservoir species. Recent identification of mpox cases in Kinshasa shows that multiple lineages circulate in a large urban center, indicating separate introduction events.
Conclusion The mpox epidemic in the DRC exhibits two distinct patterns. In traditional endemic regions, the epidemic is predominated by zoonotic spill-over events involving clade Ia. Conversely, in the eastern part of the country, the clade Ib outbreak is driven by human-to-human transmission highlighting the need for a coordinated response effort at the national, regional and international levels.
Competing Interest Statement
The authors have declared no competing interest.
Funding Statement
This study was supported by Africa CDC Pathogen Genomics Initiative (Africa PGI) (Grants: INV-018278; INV-033857; Saving Lives and Livelihoods program; NU2HGH000077); Agence Francaise de Developpement through the AFROSCREEN project (grant agreement CZZ3209, coordinated by ANRS-MIE Maladies infectieuses emergentes in partnership with Institut de Recherche pour le Developpement (IRD) and Pasteur Institute); PANAFPOX project funded by ANRS-MIE ; Belgian Directorate-general Development Cooperation and Humanitarian Aid and the Research Foundation - Flanders (FWO, grant number G096222 N to L.L.); Department of Defense, Defense Threat Reduction Agency, Monkeypox Threat Reduction Network; USDA Non-Assistance Cooperative Agreement #20230048; International mpox Research Consortium (IMReC) through funding from the Canadian Institutes of Health Research and International Development Research Centre (grant no. MRR-184813); Wellcome Trust (Collaborators Award 206298/Z/17/Z, ARTIC network); E.L. received a PhD grant from the French Foreign Office.
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
The details of the IRB/oversight body that provided approval or exemption for the research described are given below:
Ethics Committee of Kinshasa School of Public Health, University of Kinshasa (ESP-UNIKIN, Number ESP/CE/05/2023) gave ethical approval for this work
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
↵ ** Jointly supervised
Conflict of Interest: The authors declare to have no conflict of interest.
authors and their affiliation updated, Introduction, figure 2 revised, figures legend updated.
Data Availability
All data produced in the present study are available upon reasonable request to the authors
View the discussion thread.
Supplementary Material
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Infectious Diseases (except HIV/AIDS)
- Addiction Medicine (343)
- Allergy and Immunology (666)
- Anesthesia (180)
- Cardiovascular Medicine (2630)
- Dentistry and Oral Medicine (314)
- Dermatology (222)
- Emergency Medicine (397)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (932)
- Epidemiology (12183)
- Forensic Medicine (10)
- Gastroenterology (756)
- Genetic and Genomic Medicine (4071)
- Geriatric Medicine (387)
- Health Economics (679)
- Health Informatics (2628)
- Health Policy (998)
- Health Systems and Quality Improvement (981)
- Hematology (362)
- HIV/AIDS (845)
- Infectious Diseases (except HIV/AIDS) (13662)
- Intensive Care and Critical Care Medicine (792)
- Medical Education (399)
- Medical Ethics (109)
- Nephrology (431)
- Neurology (3840)
- Nursing (209)
- Nutrition (571)
- Obstetrics and Gynecology (735)
- Occupational and Environmental Health (692)
- Oncology (2011)
- Ophthalmology (582)
- Orthopedics (239)
- Otolaryngology (304)
- Pain Medicine (250)
- Palliative Medicine (74)
- Pathology (471)
- Pediatrics (1109)
- Pharmacology and Therapeutics (460)
- Primary Care Research (449)
- Psychiatry and Clinical Psychology (3406)
- Public and Global Health (6504)
- Radiology and Imaging (1390)
- Rehabilitation Medicine and Physical Therapy (808)
- Respiratory Medicine (869)
- Rheumatology (401)
- Sexual and Reproductive Health (407)
- Sports Medicine (341)
- Surgery (441)
- Toxicology (52)
- Transplantation (185)
- Urology (165)

COMMENTS
Currently Has Open Funding Opportunities. Defense Medical Research and Development Medical Simulation and Information Sciences Research Program (JPC-1) Military Infectious Diseases Research Program (JPC-2) Military Operational Medicine Research Program (JPC-5) Combat Casualty Care Research Program (JPC-6) Radiation Health Effects Research ...
Open Funding Opportunities. » Click on Image to View Funding Opportunities Brochure. Funding Opportunities now available: Alcohol and Substance Use Disorders. Arthritis. Breast Cancer. Combat Readiness-Medical. Defense Medical Research and Development. Kidney Cancer.
DEPARTMENT OF DEFENSE - CONGRESSIONALLY DIRECTED MEDICAL RESEARCH PROGRAMS. Contact Us ... To view past Program Funding Opportunities, please visit the Program Funding Opportunities Archives. ... Fiscal Year 2024 (FY24) Defense Medical Research and Development Program (DMRDP) is currently accepting applications for one award mechanism.
Medical Research Program (PRMRP). For FY2024, $370 million was appropriated for the PRMRP. PRMRP funding supports grants for medical research on a number of conditions or treatment. modalities that are of "clear scientific merit and direct relevance to military health." Congress specifies an annual list of eligible conditions or treatments.
Congressionally Directed Medical Research Programs (CDMRP). The U.S. Army Medical Research and Development Command (MRDC), in coordination with the Defense Health Agency, administers the program using a competitive grant process. CDMRP funding is to be used only for medical research on congressionally identified medical research topics (e.g ...
Combat Casualty Care Research Program (August Pre-app deadline) Chronic Pain Management Research Program (August Pre-app deadline) Toxic Exposures Research Program (September Pre-app deadlines) More programs to be posted soon. The most up to date information may be found on the DoD Funding Opportunities webpage.
DOD uses a cyclical, routine process to prioritize investments from these funds. In FYs 2015-19, it distributed nearly 100% of its $4.46 billion in funding. DOD coordinates with the Department of Veterans Affairs and the National Institutes of Health, which also sponsor research.
The Department of Defense's (DoD's) Congressionally Directed Medical Research Programs (CDMRP) has a well-established process for managing the review and selection of funding applications that it receives for its medical research programs. This chapter presents an overview of that process and provides a brief overview of CDMRP's organization and structure. The current functions of the program ...
For FY2021, $370 million was appropriated for the PRMRP. PRMRP funding supports grants for medical research on a number of conditions or treatment modalities that are of "clear scientific merit and direct relevance to military health.". Congress specifies an annual list of eligible conditions or treatments that typically includes a few ...
The David L. Boren National Security Education Act of 1991 mandated that the Secretary of Defense create and sustain a program to award scholarships to U.S. undergraduate students, fellowships to U.S. graduate students, and grants to U.S. institutions of higher education. These awards are for study or program development in languages and ...
DEPARTMENT OF DEFENSE - CONGRESSIONALLY DIRECTED MEDICAL RESEARCH PROGRAMS. Contact Us ... Defense Medical Research and Development. Medical Simulation and Information Sciences Research Program (JPC-1) ... Program Funding Opportunities FY24 Arthritis Research Program (ATRP)
About CDMRP. Since 1992, Congress has provided funding for the Congressionally Directed Medical Research Programs (CDMRPs) in the annual Defense Appropriations Act. Specific funding levels are provided for disease-specific programs, ranging from more common cancers to rare diseases to Gulf War Illness.
The medical research landscape in the United States is supported by a variety of organizations that spend billions of dollars in government and private funds each year to seek answers to complex medical and public health problems. The largest government funder is the National Institutes of Health (NIH), followed by the Department of Defense (DoD). Almost half of DoD's medical research funding ...
Medical Research Programs (CDMRP) at the U.S. Army Medical Research and Development Command (USAMRDC). The BCRP was initiated in FY92 to support innovative, high-impact research, with a mission of ending breast cancer for Service Members, Veterans, and the general public. Appropriations for the BCRP from FY92 through FY22 totaled $4.09 billion.
The Department of Defense Medical Research Office evolved from a long and successful partnership with the U.S. government. Numerous Mayo Clinic faculty and staff have been or are members of the U.S. military. The cornerstone of Mayo Clinic's rich heritage and commitment to military service can be traced back to the earliest days of Mayo Clinic ...
Office of the Assistant Secretary of Defense for Health Affairs (OASD [HA]) and the Defense Health Agency (DHA). The objective of the MHSR grant is to foster research capability and capacity within the Military Health System (MHS) that supports the MHS transition to an integrated health system focused on the Quadruple Aim: improved health ...
The 2024 Military Health System Research Symposium, the Department of Defense's premier scientific meeting focused on the unique medical needs of the warfighter, kicked off on Aug. 26. Skip main navigation ... Although the Defense Health Agency may or may not use these sites as additional distribution channels for Department of Defense ...
In order to create a search query, please fill in the following field (s) with data that most closely match your search criteria, then press the Perform Search button. Note: 1. Search results include only those projects assigned to CDMRP for management. 2. All projects within a given program may not be available until the award has been finalized.
The Department of Defense today announced $46.8 million in grants to 19 university-based faculty teams under its Minerva Research Initiative. These three- to five-year awards support basic research in social and behavioral sciences on topics relevant to U.S. national security.
The Defense Health Research Consortium was founded by CRD Associates to bring a diverse community together to advocate for the CDMRP, a $1.5 billion medical research program designed to meet the unique health and medical challenges of the men and women in the U.S. Armed Services. Funding for the CDMRP is added every year by Congress to the ...
Immuron Announces New U.S. Department of Defense Research Award for Naval Medical Research Command and Walter Reed Army Institute of Research to advance Travelan® ... IMRN), an Australian based and globally integrated biopharmaceutical company is pleased to announce the funding of a new research agreement for the Naval Medical Research Command ...
The Defense Department announced the award of the Defense Science, Technology, Engineering, and Mathematics Education Consortium Cooperative Agreement to the Research Triangle Institute and its ...
For FY2020, $110 million was appropriated for the PRCRP, separate from other CDMRP funding for breast, kidney, lung, melanoma, ovarian, pancreatic, prostate, and rare cancers. Table 3 lists all 14 cancers and treatments eligible for FY2020 PRCRP funding. Funding for FY2020. Table 2.
Each of these major research program areas is strategically guided by a committee, called a Joint Program Committee, or JPC, which consists of Department of Defense (DoD) and non-DoD medical and military technical experts. These experts work through coordinated efforts to translate guidance into research and development needs.
Dartmouth Health's Dartmouth Hitchcock Medical Center (DHMC) received a $27.7 million, seven-year grant from the National Institutes of Health (NIH) to fund projects that will speed the implementation of proven medical innovations as part of a national consortium of biomedical research centers.
U.S. Army Medical Research and Development Command, in coordination with the Defense Health Agency, administers the program using a competitive grant process. CDMRP funding is to be used only for medical research on congressionally identified medical research topics (e.g., breast cancer, gulf war illness, cancer, or other medical conditions).
In addition, reflecting the strong legacy of trusteeship cooperation, other U.S. federal agencies operate programs in the RMI consistent with U.S. laws, including the Department of Agriculture, Department of Health and Human Services, and Department of Education. Separately, the United States provides foreign assistance to benefit RMI.
The Consolidated Appropriations Act, 2023 provides medical research funding for the following programs managed by the Department of Defense, Congressionally Directed Medical Research Programs (CDMRP): Alcohol and Substance Use Disorders Research Program - $4.0 million;
Background: Recent reports raise concerns on the changing epidemiology of mpox in the Democratic Republic of the Congo (DRC), with increasing case counts, sexual contact-mediated clusters, and sustained human-to-human transmission driven by a novel monkeypox virus (MPXV) subclade, clade Ib. However, only a limited number of clade I MPXV genomes have been characterized so far, from a limited ...
The Peer Reviewed Medical Research Program (PRMRP), established in fiscal year 1999 (FY99), has supported research across the full range of science and medicine, with an underlying goal of enhancing the health, care, and well-being of military Service members, Veterans, retirees, and their family members. Program oversight is provided by a ...