Home — Essay Samples — Nursing & Health — Public Health Issues — Vaccination

Essays on Vaccination
Vaccines essay topics and outline examples, essay title 1: "the vital role of vaccines in public health: debunking myths and upholding science".
Thesis Statement: Vaccines are a cornerstone of public health, and it is crucial to dispel misinformation and emphasize the overwhelming scientific evidence supporting their safety and efficacy.
Essay Outline:
- Introduction
- The History and Impact of Vaccines
- Common Vaccine Myths and Misconceptions
- Scientific Evidence Supporting Vaccines
- Vaccine Safety and Adverse Effects
- The Importance of Herd Immunity
- Addressing Vaccine Hesitancy
Essay Title 2: "Vaccination Mandates: Balancing Individual Rights with Public Health"
Thesis Statement: While respecting individual rights is essential, vaccination mandates are a legitimate measure to safeguard public health and prevent outbreaks of vaccine-preventable diseases.
- The Concept of Vaccination Mandates
- Individual Rights and Autonomy
- Public Health Concerns and Disease Prevention
- Legal and Ethical Considerations
- Case Studies of Vaccine Mandates
- Opposition and Challenges to Mandates
Essay Title 3: "The Impact of Vaccine Disinformation on Public Health: A Global Challenge"
Thesis Statement: The proliferation of vaccine disinformation poses a significant threat to public health, and addressing this challenge is vital to ensure widespread vaccine acceptance and disease control.
- The Spread and Impact of Vaccine Disinformation
- Factors Contributing to Vaccine Hesitancy
- The Role of Social Media and Online Platforms
- Countering Vaccine Disinformation Efforts
- Global Initiatives and Collaborations
- Case Studies on Successful Interventions
The Issues Surrounding Vaccination and Its Importance
Arguments about the importance of making vaccinations mandatory, made-to-order essay as fast as you need it.
Each essay is customized to cater to your unique preferences
+ experts online
Vaccination – The Greatest Invention of All Times
My research of advantages and disadvantages of vaccination, the use of vaccination – a choice for every one, the problem of the vaccine war in the world, let us write you an essay from scratch.
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
The Ethical Theories and Issues Surrounding Vaccination in America
The effectiveness of vacciness in preventing illnesses and infectious diseases, the importance of vaccines to prevent infectious diseases, advantages and disadvantages of the various types of vaccines, get a personalized essay in under 3 hours.
Expert-written essays crafted with your exact needs in mind
Chickenpox: History, Symptoms and Treatment
The importance of increasing hpv vaccination in children, why is vaccination of human papillomavirus significant, debate on vaccination and autism, impact of media on parents' acceptance of immunization, the use of vaccines in modern medicine and the vaccination delimma, legal and ethical issues about the mmr vaccine, an argument in favor of using vaccines, the urgent need for a vaccine against zika virus, report on the measles disease and vaccination, yellow fever disease - what problems are caused by mosquitoes, chasing polio eradication: vaccine development, the examination of human sciences in connection to the effectiveness of vaccines, the different types of vaccines, vaccine types, should vaccinations be mandatory: future safety for children, should parents vaccinate their child, should vaccines be required to attend public school, why you should get vaccinated: a persuasive discussion, vaccination: advantages, hesitancy, and herd immunity.
Vaccination, also known as immunization, is a medical procedure that involves the administration of a vaccine to stimulate the immune system and provide protection against specific infectious diseases. It is a preventive measure designed to enhance the body's natural defenses by introducing harmless fragments of the disease-causing agent or weakened or inactivated forms of the pathogen.
The mechanism of vaccination involves introducing a weakened or inactivated form of a disease-causing agent, such as a virus or bacterium, into the body. This prompts the immune system to recognize and respond to the pathogen. When a vaccine is administered, it stimulates the immune system to produce an immune response, similar to what would happen during a natural infection. The immune system recognizes the foreign antigens present in the vaccine and mounts a defense by producing antibodies and activating immune cells. These immune responses help the body develop immunity against the specific pathogen. Vaccination can also involve the use of genetically engineered proteins or pieces of the pathogen to stimulate an immune response. These components are known as antigens and can be derived from the outer coats of viruses or the cell walls of bacteria. By introducing these harmless components of the pathogen into the body, vaccines help the immune system recognize and remember the specific pathogen. This way, if the individual is later exposed to the actual disease-causing agent, their immune system can mount a rapid and effective response to neutralize or eliminate the pathogen, preventing the development of the disease or reducing its severity.
1. Inactivated Vaccines 2. Live Attenuated Vaccines 3. Subunit, Recombinant, and Conjugate Vaccines 4. mRNA Vaccines 5. Viral Vector Vaccines
The origin of vaccination can be traced back to ancient times, although the concept was not fully understood at the time. The practice of vaccination, as we know it today, began with the discovery of immunization against smallpox by Edward Jenner in the late 18th century. Jenner, an English physician, observed that milkmaids who had contracted cowpox, a much milder disease, seemed to be protected against smallpox. In 1796, he conducted an experiment where he took material from a cowpox sore and inoculated it into an eight-year-old boy named James Phipps. Afterward, Jenner exposed the boy to smallpox, but he did not develop the disease. This groundbreaking experiment led to the development of the smallpox vaccine. The term "vaccination" itself comes from the Latin word "vacca," meaning cow, as the original smallpox vaccine was derived from cowpox. Jenner's work paved the way for the development of vaccines against other infectious diseases, and vaccination quickly became a widely accepted method for preventing and controlling the spread of deadly diseases.
Public opinion on vaccination varies across different societies and individuals. Overall, vaccination has been widely accepted and supported by the majority of the population, recognizing its significant role in preventing and controlling infectious diseases. Vaccines have been instrumental in eradicating or significantly reducing the impact of diseases such as smallpox, polio, measles, and more. However, there are also pockets of skepticism and opposition towards vaccination, driven by various factors such as misinformation, fear, religious beliefs, or concerns about vaccine safety. This has led to the emergence of anti-vaccine movements and vaccine hesitancy in some communities. Public opinion on vaccination is influenced by various factors, including access to accurate information, trust in healthcare professionals and scientific research, cultural and religious beliefs, personal experiences, and the influence of social media and other communication channels. Efforts to promote vaccination and address vaccine hesitancy involve public health campaigns, education, and communication strategies to provide accurate information about vaccines, address concerns, and emphasize the importance of vaccination in protecting individual and public health.
1. Disease prevention 2. Herd immunity 3. Public health impact 4. Safety and effectiveness 5. Global impact
1. Vaccine safety concerns 2. Personal freedom and choice 3. Misinformation and skepticism 4. Religious or philosophical objections 5. Perception of low disease risk
1. According to the World Health Organization (WHO), vaccines prevent between 2-3 million deaths worldwide every year. 2. Smallpox is the only disease that has been totally eradicated through vaccination. 3. Vaccines have significantly reduced the global burden of infectious diseases. For instance, measles deaths decreased by 73% worldwide between 2000 and 2018. 4. The influenza vaccine helps reduce the risk of severe illness and hospitalization. In the United States, annual flu vaccination prevented an estimated 7.5 million flu illnesses during the 2019-2020 season. 5. The average vaccine takes around 10-15 years of research and development before it is widely available.
The topic of vaccination is of paramount importance when considering the impact it has had on public health. Writing an essay about vaccination provides an opportunity to explore the profound significance of this medical intervention. Vaccination has played a pivotal role in preventing and controlling infectious diseases, saving countless lives worldwide. By delving into the subject, one can highlight the historical development of vaccines, their mechanisms of action, and the scientific evidence supporting their effectiveness. Furthermore, examining the topic of vaccination allows for an exploration of the public health implications, including the concept of herd immunity and the role of vaccination in disease eradication efforts. It also provides a platform to address the various arguments surrounding vaccine hesitancy and vaccine refusal, shedding light on the importance of accurate information, education, and communication. Moreover, the essay can delve into the ethical considerations surrounding vaccination policies, such as balancing individual autonomy with the collective responsibility for public health. By exploring these aspects, one can foster a deeper understanding of the challenges, controversies, and potential solutions in promoting vaccination uptake.
1. American Academy of Pediatrics. (2018). Immunization information for parents. https://www.healthychildren.org/English/safety-prevention/immunizations/Pages/default.aspx 2. Centers for Disease Control and Prevention. (2021). Vaccines & immunizations. https://www.cdc.gov/vaccines/index.html 3. Gust, D. A., Darling, N., Kennedy, A., & Schwartz, B. (2008). Parents with doubts about vaccines: Which vaccines and reasons why. Pediatrics, 122(4), 718-725. https://doi.org/10.1542/peds.2007-0538 4. Larson, H. J., de Figueiredo, A., Xiahong, Z., Schulz, W. S., Verger, P., Johnston, I. G., Cook, A. R., Jones, N. S., & the SAGE Working Group on Vaccine Hesitancy. (2016). The state of vaccine confidence 2016: Global insights through a 67-country survey. EBioMedicine, 12, 295-301. https://doi.org/10.1016/j.ebiom.2016.08.042 5. MacDonald, N. E., Hesitancy SAGE Working Group. (2015). Vaccine hesitancy: Definition, scope and determinants. Vaccine, 33(34), 4161-4164. https://doi.org/10.1016/j.vaccine.2015.04.036 6. Offit, P. A., Quarles, J., Gerber, M. A., Hackett, C. J., & Marcuse, E. K. (2002). Addressing parents' concerns: Do vaccines cause allergic or autoimmune diseases? Pediatrics, 110(6), 1113-1116. https://doi.org/10.1542/peds.110.6.1113 7. Omer, S. B., Salmon, D. A., Orenstein, W. A., deHart, M. P., & Halsey, N. (2009). Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. New England Journal of Medicine, 360(19), 1981-1988. https://doi.org/10.1056/NEJMsa0806477 8. Smith, P. J., Humiston, S. G., Parnell, T., Vannice, K. S., & Salmon, D. A. (2011). The association between intentional delay of vaccine administration and timely childhood vaccination coverage. Public Health Reports, 126(Suppl 2), 135-146. https://doi.org/10.1177/00333549111260S219 9. World Health Organization. (2019). Ten threats to global health in 2019. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 10. World Health Organization. (2021). Immunization coverage. https://www.who.int/news-room/fact-sheets/detail/immunization-coverage
Relevant topics
- Eating Disorders
- Drug Addiction
- Childhood Obesity
- Teenage Pregnancy
- Birth Control
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
Advertisement
Intermittent Fasting
Pre-Exposure Prophylaxis (PrEP)
The importance of vaccinations.
Last Updated September 2024 | This article was created by familydoctor.org editorial staff and reviewed by Deepak S. Patel, MD, FAAFP, FACSM
Related Topics
Childhood vaccines: what they are and why your child needs them, immunization schedules, preventive services for healthy living.
Name (required)
Mail (will not be published) (required)
Gimme a site! Just a username, please.
Remember Me
There has been confusion and misunderstandings about vaccines. But vaccinations are an important part of family and public health. Vaccines prevent the spread of contagious, dangerous, and deadly diseases. These include measles , polio, mumps , chicken pox , whooping cough , diphtheria, HPV , and COVID-19 .
The first vaccine discovered was the smallpox vaccine. Smallpox was a deadly illness. It killed 300 million to 500 million people around the world in the last century. After the vaccine was given to people, the disease was eventually erased. It’s the only disease to be completely destroyed. There are now others close to that point, including polio.
When vaccination rates decline, cases of preventable diseases go up. This has been happening in recent years with measles. As of July 7, 2023, the Centers for Disease Control has been notified of 18 confirmed cases in 12 U.S. jurisdictions. That may not seem like a lot but compare it with just 3 cases during the same time in 2022. By the end of 2022, there were 121 cases. Almost all those cases could have been prevented with vaccines.
What are vaccines?
A vaccine (or immunization) is a way to build your body’s natural immunity to a disease before you get sick. This keeps you from getting and spreading the disease.
For some vaccines, a weakened form of the disease germ is injected into your body. This is usually done with a shot in the leg or arm. Your body detects the invading germs (antigens) and produces antibodies to fight them. Those antibodies then stay in your body for a long time. In many cases, they stay for the rest of your life. If you’re ever exposed to the disease again, your body will fight it off without you ever getting the disease.
Some illnesses, like strains of cold viruses, are fairly mild. But some, like COVID-19, smallpox or polio, can cause life-altering changes. They can even result in death. That’s why preventing your body from contracting these illnesses is very important.
How does immunity work?
Your body builds a defense system to fight foreign germs that could make you sick or hurt you. It’s called your immune system. To build up your immune system, your body must be exposed to different germs. When your body is exposed to a germ for the first time, it produces antibodies to fight it. But that takes time, and you usually get sick before the antibodies have built up. But once you have antibodies, they stay in your body. So, the next time you’re exposed to that germ, the antibodies will attack it, and you won’t get sick.
Path to improved health
Everyone needs vaccines. They are recommended for infants, children, teenagers, and adults. There are widely accepted immunization schedules available. They list what vaccines are needed, and at what age they should be given. Most vaccines are given to children. It’s recommended they receive 12 different vaccines by their 6th birthday. Some of these come in a series of shots. Some vaccines are combined so they can be given together with fewer shots.
The American Academy of Family Physicians (AAFP) believes that immunization is essential to preventing the spread of contagious diseases. Vaccines are especially important for at-risk populations such as young children and older adults. The AAFP offers vaccination recommendations, immunization schedules , and information on disease-specific vaccines.
Being up to date on vaccines is especially important as children head back to school. During the 2021 school year, state-required vaccines among kindergarteners dropped from 95% to 94%. In the 2021-2022 year it fell again to 93%. Part of this was due to disruptions from the COVID-19 pandemic.
Is there anyone who can’t get vaccines?
Some people with certain immune system diseases should not receive some types of vaccines and should speak with their health care providers first. There is also a small number of people who don’t respond to a particular vaccine. Because these people can’t be vaccinated, it’s very important everyone else gets vaccinated. This helps preserve the “herd immunity” for the vast majority of people. This means that if most people are immune to a disease because of vaccinations, it will stop spreading.
Are there side effects to vaccines?
There can be side effects after you or your child get a vaccine. They are usually mild. They include redness or swelling at the injection site. Sometimes children develop a low-grade fever. These symptoms usually go away in a day or two. More serious side effects have been reported but are rare.
Typically, it takes years of development and testing before a vaccine is approved as safe and effective. However, in cases affecting a global, public health crisis or pandemic, it is possible to advance research, development, and production of a vaccine for emergency needs. Scientists and doctors at the U.S. Food and Drug Administration (FDA) study the research before approving a vaccine. They also inspect places where the vaccines are produced to make sure all rules are being followed. After the vaccine is released to the public, the FDA continues to monitor its use. It makes sure there are no safety issues.
The benefits of their use far outweigh any risks of side effects.
What would happen if we stopped vaccinating children and adults?
If we stopped vaccinating, the diseases would start coming back. Aside from smallpox, all other diseases are still active in some part of the world. If we don’t stay vaccinated, the diseases will come back. There would be epidemics, just like there used to be.
This happened in Japan in the 1970s. They had a good vaccination program for pertussis (whooping cough). Around 80% of Japanese children received a vaccination. In 1974, there were 393 cases of whooping cough and no deaths. Then rumors began that the vaccine was unsafe and wasn’t needed. By 1976, the vaccination rate was 10%. In 1979, there was a pertussis epidemic, with more than 13,000 cases and 41 deaths. Soon after, vaccination rates improved, and the number of cases went back down.
Things to consider
There have been many misunderstandings about vaccines. There are myths and misleading statements that spread on the internet and social media about vaccines. Here are answers to 5 of the most common questions/misconceptions about vaccines.
Vaccines do NOT cause autism.
Though multiple studies have been conducted, none have shown a link between autism and vaccines. The initial paper that started the rumor has since been discredited.
Vaccines are NOT too much for an infant’s immune system to handle.
Infants’ immune systems can handle much more than what vaccines give them. They are exposed to hundreds of bacteria and viruses every day. Adding a few more with a vaccine doesn’t add to what their immune systems are capable of handling.
Vaccines do NOT contain toxins that will harm you.
Some vaccines contain trace amounts of substances that could be harmful in a large dose. These include formaldehyde, aluminum, and mercury. But the amount used in the vaccines is so small that the vaccines are completely safe. For example, over the course of all vaccinations by the age of 2, a child will take in 4mg of aluminum. A breast-fed baby will take in 10mg in 6 months. Soy-based formula delivers 120mg in 6 months. In addition, infants have 10 times as much formaldehyde naturally occurring in their bodies than what is contained in a vaccine. And the toxic form of mercury has never been used in vaccines.
Vaccines do NOT cause the diseases they are meant to prevent.
This is a common misconception, especially about the flu vaccine. Many people think they get sick after getting a flu shot. But flu shots contain dead viruses—it’s impossible to get sick from the shot but mild symptoms can occur because the vaccine may trigger an immune response, which is normal. Even with vaccines that use weakened live viruses, you could experience mild symptoms similar to the illness. But you don’t actually have the disease.
We DO still need vaccines in the U.S., even though infection rates are low.
Many diseases are uncommon in the U.S. because of our high vaccination rate. But they haven’t been eliminated from other areas of the world. If a traveler from another country brings a disease to the U.S., anyone who isn’t vaccinated is at risk of getting that disease. The only way to keep infection rates low is to keep vaccinating.
Questions to ask your doctor
- Why does my child need to be vaccinated?
- What are the possible side effects of the vaccination?
- What do I do if my child experiences a side effect from the vaccine?
- What happens if my child doesn’t get all doses of the recommended vaccines? Will he or she be able to go to daycare or school?
- We missed a vaccination. Can my child still get it late?
- Are there new vaccines that aren’t on the immunization schedules for kids?
- What should I do if I don’t have health insurance, or my insurance doesn’t cover vaccinations?
- What vaccinations do I need as an adult?
- Why do some people insist they became sick after getting the flu vaccine?
Centers for Disease Control and Prevention: Vaccines & Immunizations
Last Updated: September 6, 2024
This article was contributed by familydoctor.org editorial staff.
Copyright © American Academy of Family Physicians
This information provides a general overview and may not apply to everyone. Talk to your family doctor to find out if this information applies to you and to get more information on this subject.
Related Articles
Most schools and daycares require certain childhood vaccines, but they’re also good for your child’s health.
Certain immunizations are important at every age to reduce illness, hospitalization, or death.
A preventive service might be a test, an immunization or vaccine, or advice from your doctor. These services can…

familydoctor.org is powered by

Visit our interactive symptom checker

The Childhood Immunization Schedule and Safety: Stakeholder Concerns, Scientific Evidence, and Future Studies (2013)
Chapter: 7 conclusions and recommendations.
Conclusions and Recommendations
COMMITTEE RESPONSE TO ITS STATEMENT OF TASK
This final chapter highlights selected findings and conclusions and presents recommendations for each section of the committee’s statement of task. The preceding chapters, especially Chapter 6 , include many assessments that may be construed as the committee’s preferences among the alternatives presented but that fall short of formal recommendations.
Vaccine safety is critically important, but a determination of safety is ultimately a value judgment. For example, some might believe that a serious adverse event that occurs once in 1 million doses is “safe enough” relative to the benefit of preventing a serious disease, whereas others may consider that risk unacceptably high. The committee did not set a specific numerical target or goal for what should be considered “safe enough.” Instead, the committee made a judgment based on the literature that failed to link adverse effects to schedule exposures or multiple immunizations, concluding that there is no evidence that the schedule is not safe.
The committee recognized that final decisions about research studies must await knowledge of further evidence, including biological plausibility and/or epidemiological evidence, feasibility, cost, and the exact circumstances of stakeholder concerns, before the planning and conduct of specific research projects. In turn, the committee believes that it would be inappropriate to make unqualified recommendations without this knowledge. The committee notes that stakeholder concerns may be used to drive a search for scientific evidence (biological or epidemiological), although such concerns would not be sufficient motivation to embark
on costly clinical research, such as new randomized controlled trials or cohort studies.
The committee thus decided to make five general recommendations. Three recommendations focus on improvements to understanding stakeholder concerns, harmonizing research methods, and sequencing the process for selecting research questions. Two recommendations focus on research methods, including randomized controlled trials and data systems that would enable ongoing and improved observational studies.
Statement of Task (Part I): Review scientific findings and stakeholder concerns related to the safety of the recommended childhood immunization schedule.
Summary of Stakeholder Concerns
The committee’s findings and conclusions about stakeholder concerns are presented in Chapter 4 . Although the committee identified the concerns of some parents about the number, frequency, and timing of immunizations in the overall immunization schedule, the committee did not find in its literature review that clinicians, public health personnel, or policy makers have similar safety concerns. Among the latter groups, the childhood immunization schedule is considered to be among the most effective and safe of the public interventions available to prevent serious disease and death. However, although health care professionals have much information about individual vaccines, they have much less information about the effects of administration of multiple vaccines at a single visit or the timing of the immunizations. Additionally, the cited concerns of health care professionals include efficacy of certain vaccines as well as appropriate delivery and communication regarding the recommended childhood immunization schedule.
Although the 2010 National Vaccine Plan addresses the need to provide health care providers with more timely, accurate, and transparent information about the benefits and risks of vaccines, the plan does not specifically address strategies to assist providers with questions about the safety of the immunization schedule (HHS, 2010). The committee concluded that parents and health care professionals would benefit from more comprehensive and detailed information with which to address parental concerns about the safety of the immunization schedule. Such information should clearly address vaccine-preventable diseases, the risks and benefits of immunizations, and the safety of the immunization schedule.
The committee’s literature review highlighted the lack of high-quality evidence supporting stakeholder concerns (the priority stakeholders are listed in Box 4-1 ) about the immunization schedule. In its role to ensure
vaccine safety, the federal government has already prioritized the engagement of stakeholders in multiple activities, as detailed in the 2010 National Vaccine Plan and implementation efforts, as well as the Centers for Disease Control and Prevention’s Immunization Safety Office scientific agenda (CDC, 2011; HHS, 2010). However, an effective national vaccine program will require more complete information on stakeholder concerns about the safety of the immunization schedule, the severity of vaccine-preventable diseases, individual- and population-level immunization rates, vaccine efficacy, and the delivery and supply of vaccines recommended in the childhood immunization schedule. Improved communication between public health authorities and parents requires improvements to the clarity of the information provided, as well as the building of trust and the use of a systematic approach to elicit public concerns. Further research into the type of questions that parents seek to answer by the use of the scientific methods of social, behavioral, and decision science is indicated.
On the basis of the committee’s literature review and public testimony, the committee strongly endorses the need for research to understand the public’s knowledge, beliefs, and concerns about vaccines and vaccine-preventable diseases in particular, which is a key strategy in the 2010 National Vaccine Plan (HHS, 2010). It must be acknowledged that the methods used in most immunization studies do not permit a detailed analysis of the impact of parental concerns on the decision to immunize their children. Although the committee found that the largest safety concerns exist among a subset of parents, the concerns of multiple stakeholders should be included as part of the efforts of the National Vaccine Program Office (NVPO). For example, health care providers have much knowledge about individual vaccines but less information about the effects of administering multiple vaccines at a single visit or the timing of the immunizations.
Recommendation 4-1: The committee recommends that the National Vaccine Program Office systematically collect and assess evidence regarding public confidence in and concerns about the entire childhood immunization schedule, with the goal to improve communication with health care professionals, and between health care professionals and the public regarding the safety of the schedule.
Summary of Scientific Findings
The committee’s findings and conclusions about the safety of the immunization schedule on the basis of the information in the scientific literature are presented in Chapter 5 . The committee encountered two major issues. First, the concept of the immunization “schedule” is not well developed in the scientific literature. Most vaccine research focuses on the health outcomes associated with single immunizations or combinations of vaccines
administered at a single visit. Even though each new vaccine is evaluated in the context of the overall immunization schedule that existed at the time of review, individual elements of the schedule are not evaluated once it is adjusted to accommodate a new vaccine. Key elements of the immunization schedule—for example, the number, frequency, timing, order, and age at the time of administration of vaccines—have not been systematically examined in research studies.
The second major issue that the committee encountered during the review of the scientific literature was uncertainty over whether the scientific literature has addressed all health outcomes and safety concerns. The committee could not determine whether its list of health outcomes was complete or whether a more comprehensive system of surveillance might identify other outcomes of potential safety significance. In addition, the conditions of concern to some stakeholders, such as immunological, neurological, and developmental problems, are illnesses and conditions for which the etiology, in general, is not well understood. Further research on these conditions may clarify their etiologies.
Finally, the committee found that evidence from assessments of health outcomes in potentially susceptible subpopulations of children who may have an increased risk of adverse reactions to vaccines (such as children with a family history of autoimmune disease or allergies or children born prematurely) was limited and is characterized by uncertainty about the definition of populations of interest and definitions of exposures and outcomes. Most children who experience an adverse reaction to immunization have a preexisting susceptibility. Some predispositions may be detectable prior to vaccination; others, at least with current technology and practice, are not (IOM, 2012, p. 82).
In summary, to consider whether and how to study the safety and health outcomes of the entire childhood immunization schedule, the field needs valid and accepted metrics of the entire immunization schedule (the “exposure”) and clearer definitions of health outcomes linked to stakeholder concerns (the “outcomes”) in rigorous research that will ensure validity and generalizability.
Recommendation 5-1: To improve the utility of studies of the entire childhood immunization schedule, the committee recommends that the National Vaccine Program Office develop a framework that clarifies and standardizes definitions of
- key elements of the schedule ,
- relevant health outcomes, and
- populations that are potentially susceptible to adverse events.
Statement of Task (Part II): Identify potential research approaches, methodologies, and study designs that could inform this question, including an assessment of the potential strengths and limitations of each approach, methodology, and design, as well as the financial and ethical feasibility of doing them.
Summary of Methodological Issues
The committee’s findings and conclusions about research approaches are presented in Chapter 6 . The committee parsed the phrase “this question” in Part 2 of the statement of task into four broad research questions in Box 7-1 .
The committee then discussed general research approaches with the potential to answer these questions: ongoing research with data from existing data systems, research with enhanced data from existing data systems, prospective observational studies, and randomized controlled trials. The committee also recognized that to advance the knowledge about the safety
BOX 7-1 Leading Research Questions of Interest to Select Stakeholders
- How do child health outcomes compare between those who receive no vaccinations and those who receive the full currently recommended immunization schedule?
- How do child health outcomes compare between (a) those who receive the full currently recommended immunization schedule and (b) those who omit specific vaccines?
- For children who receive the currently recommended immunization schedule, do short- or long-term health outcomes differ for those who receive fewer immunizations per visit (e.g., when immunizations are spread out over multiple occasions), or for those who receive their immunizations at later ages but still within the recommended ranges?
- Do potentially susceptible subpopulations—for example, children from families with a history of allergies or autoimmune diseases— who may experience adverse health consequences in association with immunization with the currently recommended immunization schedule exist?
of the immunization schedule, certain enhancements to the research infrastructure will be needed, as detailed in Chapter 6 .
The committee recognizes that the establishment of priorities for research will be a challenge. Thus, the committee proposes a process for setting priorities that recognizes stakeholder concerns and establishes these priorities on the basis of epidemiological and other evidence (based on formal systematic reviews), biological plausibility, and feasibility.
Before the U.S. Department of Health and Human Services (HHS) initiates further research on the entire immunization schedule through its agencies—most notably CDC, FDA, the National Institutes of Health, and NVPO—the biological plausibility of the association of a particular outcome with an aspect of the immunization schedule must be thoroughly reviewed. Along these lines, previous IOM vaccine safety committees have assessed the mechanisms by which vaccines potentially cause adverse events by identifying and evaluating the clinical and biological evidence (from human, animal, and in vitro studies) for individual vaccines. Furthermore, the recent IOM Committee to Review Adverse Effects of Vaccines developed categories for a mechanistic assessment of the weight of the evidence. Each assessment considers clinical information from case reports and clinical and experimental evidence from other sources (IOM, 2012).
Recommendation 6-1: The committee recommends that the Department of Health and Human Services incorporate study of the safety of the overall childhood immunization schedule into its processes for setting priorities for research, recognizing stakeholder concerns, and establishing the priorities on the basis of epidemiological evidence, biological plausibility, and feasibility.
The decision to initiate further studies should be based on an evaluation of three considerations that the committee identified through its review of stakeholder concerns and scientific findings:
- epidemiological evidence of potential adverse health outcomes associated with elements of the immunization schedule (such as postmarketing signals or indications of elevated risk from observational studies);
- biological plausibility supporting hypotheses linking specific aspects of the immunization schedule with particular adverse health outcomes; and
- concern about the immunization schedule’s safety expressed by stakeholders, which should initiate efforts to explore the two previous considerations.
The committee acknowledges the evidence that reducing vaccine coverage is associated with increases in vaccine-preventable disease and found only inconsistent and anecdotal evidence to imply that the recommended immunization schedule is not safe. Furthermore, existing systems for the detection of adverse events provide confidence that the existing childhood immunization schedule is safe, and the committee recognizes that the federal government invests considerable resources to ensure vaccine safety. Nevertheless, some stakeholders have suggested that further work is warranted, such as a comparison of vaccinated children with unvaccinated children or children receiving immunizations on alternative immunization schedules.
The committee supports the National Vaccine Advisory Committee Safety Working Group statement that “the strongest study design, a prospective, randomized clinical trial that includes a study arm receiving no vaccine or vaccine not given according to the current recommended schedule, would be unethical and therefore cannot be done” (NVAC, 2009, p. 38). In Chapter 6 , the committee presents the formidable ethical and feasibility problems associated with the conduct of randomized controlled trials of children who receive all recommended immunizations and children who receive none of them and randomized controlled trials of children who receive all recommended immunizations and children who receive the recommended immunization on an alternative schedule. There are very low observed rates of adverse events with vaccination, which is another factor sffecting feasibility of a randomized controlled trial. Because of these problems, the committee concludes that a randomized controlled trial comparing the recommended schedule with any alternative schedule would be unethical and infeasible and could increase the risk of vaccine-preventable diseases in individuals and in the community.
Furthermore, the committee found that a trial of a modified version of the ACIP schedule—one that would disperse the timing of vaccinations so that children are visiting health care professionals more often but receiving fewer shots at each visit—would be ethical; however, it would add substantial costs to both parents and providers and, moreover, may be unacceptable to insurers if its effectiveness—measured as a decreased rate of adverse safety outcomes—was negligible. This modified schedule would provide immunizations within the time intervals approved by ACIP and would address the concern about immunization with too many vaccines at one office visit, but the committee did not view this option to be feasible for study.
In light of the ethical and feasibility requirements and the available evidence, the committee concludes that new randomized controlled trials of the childhood immunization schedule are not justified at this time.
Recommendation 6-2: The Department of Health and Human Services should refrain from initiating randomized controlled trials of the childhood
immunization schedule that compare safety outcomes in fully vaccinated children with those in unvaccinated children or those vaccinated by use of an alternative schedule.
The committee also reviewed opportunities to study groups that choose not to vaccinate their children by use of a prospective cohort study design. However, such a study would not conclusively reveal differences in health outcomes between unimmunized and fully immunized children for two main reasons. First, the sample populations often suggested for study (such as some religious populations) may be too small to adequately power such a comparative analysis, particularly for very rare adverse health outcomes. Such a study would also need to account for the many confounding variables that separate these naturally occurring unimmunized populations from the average U.S. child, including lifestyle factors and genetic variables.
The committee finds that secondary analyses of existing systems are more promising approaches to examination of the research questions that the committee identified in future studies of the childhood immunization schedule. The Vaccine Safety Datalink (VSD) is a useful collaborative project that could conduct both postmarketing surveillance and longer-term targeted research. The ability to augment routinely collected administrative data in VSD with data from parent interviews and reviews of medical records for a selected study population is an important strength.
VSD is currently the best available system for studying the safety of the immunization schedule in the United States. VSD should strive to improve the generalizability of its data to the U.S. population as a whole by enhancing the quality of its demographic information and by expanding its scope to include more diversity in its study populations. Secondary analyses with data from other existing databases (that might be modeled on VSD) could be a feasible, ethical, and cost-effective means of investigating several research questions that the committee identified. The committee recognizes that the commitment to VSD studies by the managed care organizations currently receiving funding through VSD needs to be sustained to continue to build on existing efforts. The committee concludes that VSD is a valuable component of the federal research infrastructure and will be the best-suited source of data for studying the childhood immunization schedule. Its utility will be expanded with the addition of more detailed demographic data and family medical histories.
Recommendation 6-3: The committee recommends that the Department of Health and Human Services (HHS) and its partners continue to fund and support the Vaccine Safety Datalink project to study the safety of the recommended immunization schedule. Furthermore, HHS should
consider expanding the collaboration with new health plan members and enhancing the data to improve its utility and generalizability.
CONCLUDING OBSERVATIONS
The committee’s efforts to identify priorities for recommended research studies did not reveal a base of evidence suggesting that the childhood immunization schedule is linked to autoimmune diseases, asthma, hypersensitivity, seizures or epilepsy, child developmental disorders, learning disorders or developmental disorders, or attention deficit or disruptive behavior disorders. While the committee found that there is no scientific evidence to justify the majority of safety concerns, perceptions dictate parental support and actions. Therefore further study of the full immunization schedule as well as further study to understand stakeholder perceptions and how they are formed may help improve awareness and education efforts. Stakeholder concerns should be one of the elements used to drive searches for scientific evidence, but these concerns alone, absent epidemiological or biological evidence, do not warrant the initiation of new high-cost randomized controlled trials. The committee concludes that data from existing data systems may be used to conduct observational studies and offer the best means for ongoing research efforts of the immunization schedule’s safety.
The committee found no significant evidence to imply that the recommended immunization schedule is not safe. Furthermore, existing surveillance and response systems have identified adverse events known to be associated with vaccination. The federal immunization research infrastructure is strong. A key component is the VSD project, which with ongoing support will be able to feasibly address the committee’s identified key research questions. Although the committee concludes that protection of children from vaccine-preventable diseases is of higher importance than testing of alternative immunization schedules without epidemiological or biological evidence indicating a safety problem, VSD should continue to examine the health outcomes of people who choose alternative schedules.
Looking to the future, the committee supports the work of the federal research infrastructure in ensuring that stakeholders are involved in all stages of development, implementation, evaluation, and dissemination of the immunization schedule. As electronic medical records become more commonly used, they may provide an opportunity to capture complete immunization data linked with hospital discharge records that will be useful to future studies. Further, the Post-Licensure Rapid Immunization Safety Monitoring (PRISM) program may have the capability to monitor rare adverse events potentially associated with the childhood immunization schedule. Initiatives such as the National Children’s Study also hold promise; it
will be one of the most comprehensive research efforts focused on studying children’s health and development.
The childhood immunization schedule may become more complex over time as scientific advances are made and new vaccines are developed. Feasible research approaches to study potential adverse health outcomes will emerge only with a sustained and substantial federal commitment to research on vaccine safety.
CDC (Centers for Disease Control and Prevention). 2011. Immunization Safety Office scientific agenda. Atlanta, GA: Immunization Safety Office, Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention.
HHS (Department of Health and Human Services). 2010. 2010 National Vaccine Plan. Washington, DC: Department of Health and Human Services.
IOM (Institute of Medicine). 2012. Adverse effects of vaccines: Evidence and causality . Washington, DC: The National Academies Press.
NVAC (National Vaccine Advisory Committee). 2009. Recommendations on the Centers for Disease Control and Prevention Immunization Safety Office draft 5-year scientific agenda. Washington, DC: National Vaccine Advisory Committee.
Vaccines are among the most safe and effective public health interventions to prevent serious disease and death. Because of the success of vaccines, most Americans today have no firsthand experience with such devastating illnesses as polio or diphtheria. Health care providers who vaccinate young children follow a schedule prepared by the U.S. Advisory Committee on Immunization Practices. Under the current schedule, children younger than six may receive as many as 24 immunizations by their second birthday. New vaccines undergo rigorous testing prior to receiving FDA approval; however, like all medicines and medical interventions, vaccines carry some risk.
Driven largely by concerns about potential side effects, there has been a shift in some parents' attitudes toward the child immunization schedule. The Childhood Immunization Schedule and Safety identifies research approaches, methodologies, and study designs that could address questions about the safety of the current schedule.
This report is the most comprehensive examination of the immunization schedule to date. The IOM authoring committee uncovered no evidence of major safety concerns associated with adherence to the childhood immunization schedule. Should signals arise that there may be need for investigation, however, the report offers a framework for conducting safety research using existing or new data collection systems.
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
Now Available on Whatsapp:
+1 (888) 687-4420
Online 24/7
- College Essay
- Argumentative Essay
- Expository Essay
- Narrative Essay
- Descriptive Essay
- Scholarship Essay
- Admission Essay
- Reflective Essay
- Nursing Essay
- Economics Essay
Assignments
- Term Papers
- Research Papers
- Case Studies
- Dissertation
- Presentation
- Editing Help
- Cheap Essay Writing
- How to Order
Persuasive Essay Guide
Persuasive Essay About Covid19
How to Write a Persuasive Essay About Covid19 | Examples & Tips
14 min read

People also read
A Comprehensive Guide to Writing an Effective Persuasive Essay
A Catalogue of 300 Best Persuasive Essay Topics for Students
Persuasive Essay Outline - A Complete Guide
30+ Persuasive Essay Examples To Get You Started
Read Excellent Examples of Persuasive Essay About Gun Control
How To Write A Persuasive Essay On Abortion
Learn to Write a Persuasive Essay About Business With 5 Best Examples
Check Out 14 Persuasive Essays About Online Education Examples
Persuasive Essay About Smoking - Making a Powerful Argument with Examples
Are you looking to write a persuasive essay about the Covid-19 pandemic?
Writing a compelling and informative essay about this global crisis can be challenging. It requires researching the latest information, understanding the facts, and presenting your argument persuasively.
But don’t worry! with some guidance from experts, you’ll be able to write an effective and persuasive essay about Covid-19.
In this blog post, we’ll outline the basics of writing a persuasive essay . We’ll provide clear examples, helpful tips, and essential information for crafting your own persuasive piece on Covid-19.
Read on to get started on your essay.
- 1. Steps to Write a Persuasive Essay About Covid-19
- 2. Examples of Persuasive Essay About COVID-19
- 3. Examples of Persuasive Essay About COVID-19 Vaccine
- 4. Examples of Persuasive Essay About COVID-19 Integration
- 5. Examples of Argumentative Essay About Covid 19
- 6. Examples of Persuasive Speeches About Covid-19
- 7. Tips to Write a Persuasive Essay About Covid-19
- 8. Common Topics for a Persuasive Essay on COVID-19
Steps to Write a Persuasive Essay About Covid-19
Here are the steps to help you write a persuasive essay on this topic, along with an example essay:
Step 1: Choose a Specific Thesis Statement
Your thesis statement should clearly state your position on a specific aspect of COVID-19. It should be debatable and clear. For example:
Step 2: Research and Gather Information
Collect reliable and up-to-date information from reputable sources to support your thesis statement. This may include statistics, expert opinions, and scientific studies. For instance:
- COVID-19 vaccination effectiveness data
- Information on vaccine mandates in different countries
- Expert statements from health organizations like the WHO or CDC
Step 3: Outline Your Essay
Create a clear and organized outline to structure your essay. A persuasive essay typically follows this structure:
- Introduction
- Background Information
- Body Paragraphs (with supporting evidence)
- Counterarguments (addressing opposing views)
Step 4: Write the Introduction
In the introduction, grab your reader's attention and present your thesis statement. For example:
Step 5: Provide Background Information
Offer context and background information to help your readers understand the issue better. For instance:
Step 6: Develop Body Paragraphs
Each body paragraph should present a single point or piece of evidence that supports your thesis statement. Use clear topic sentences , evidence, and analysis. Here's an example:
Step 7: Address Counterarguments
Acknowledge opposing viewpoints and refute them with strong counterarguments. This demonstrates that you've considered different perspectives. For example:
Step 8: Write the Conclusion
Summarize your main points and restate your thesis statement in the conclusion. End with a strong call to action or thought-provoking statement. For instance:
Step 9: Revise and Proofread
Edit your essay for clarity, coherence, grammar, and spelling errors. Ensure that your argument flows logically.

Step 10: Cite Your Sources
Include proper citations and a bibliography page to give credit to your sources.
Remember to adjust your approach and arguments based on your target audience and the specific angle you want to take in your persuasive essay about COVID-19.

Paper Due? Why Suffer? That's our Job!
Examples of Persuasive Essay About COVID-19
When writing a persuasive essay about the COVID-19 pandemic, it’s important to consider how you want to present your argument. To help you get started, here are some example essays for you to read:
Here is another example explaining How COVID-19 has changed our lives essay:
Let’s look at another sample essay:
Check out some more PDF examples below:
Persuasive Essay About Covid-19 Pandemic
Sample Of Persuasive Essay About Covid-19
Persuasive Essay About Covid-19 In The Philippines - Example
If you're in search of a compelling persuasive essay on business, don't miss out on our “ persuasive essay about business ” blog!
Examples of Persuasive Essay About COVID-19 Vaccine
Covid19 vaccines are one of the ways to prevent the spread of COVID-19, but they have been a source of controversy. Different sides argue about the benefits or dangers of the new vaccines. Whatever your point of view is, writing a persuasive essay about it is a good way of organizing your thoughts and persuading others.
A persuasive essay about the COVID-19 vaccine could consider the benefits of getting vaccinated as well as the potential side effects.
Below are some examples of persuasive essays on getting vaccinated for Covid-19.
Covid19 Vaccine Persuasive Essay
Persuasive Essay on Covid Vaccines
Interested in thought-provoking discussions on abortion? Read our persuasive essay about abortion blog to eplore arguments!
Examples of Persuasive Essay About COVID-19 Integration
Covid19 has drastically changed the way people interact in schools, markets, and workplaces. In short, it has affected all aspects of life. However, people have started to learn to live with Covid19.
Writing a persuasive essay about it shouldn't be stressful. Read the sample essay below to get an idea for your own essay about Covid19 integration.
Persuasive Essay About Working From Home During Covid19
Searching for the topic of Online Education? Our persuasive essay about online education is a must-read.
Examples of Argumentative Essay About Covid 19
Covid-19 has been an ever-evolving issue, with new developments and discoveries being made on a daily basis.
Writing an argumentative essay about such an issue is both interesting and challenging. It allows you to evaluate different aspects of the pandemic, as well as consider potential solutions.
Here are some examples of argumentative essays on Covid19.
Argumentative Essay About Covid19 Sample
Argumentative Essay About Covid19 With Introduction Body and Conclusion
Looking for a persuasive take on the topic of smoking? You'll find it all related arguments in out Persuasive Essay About Smoking blog!
Examples of Persuasive Speeches About Covid-19
Do you need to prepare a speech about Covid19 and need examples? We have them for you!
Persuasive speeches about Covid-19 can provide the audience with valuable insights on how to best handle the pandemic. They can be used to advocate for specific changes in policies or simply raise awareness about the virus.
Check out some examples of persuasive speeches on Covid-19:
Persuasive Speech About Covid-19 Example
Persuasive Speech About Vaccine For Covid-19
You can also read persuasive essay examples on other topics to master your persuasive techniques!
Tips to Write a Persuasive Essay About Covid-19
Writing a persuasive essay about COVID-19 requires a thoughtful approach to present your arguments effectively.
Here are some tips to help you craft a compelling persuasive essay on this topic:
- Choose a Specific Angle: Narrow your focus to a specific aspect of COVID-19, like vaccination or public health measures.
- Provide Credible Sources: Support your arguments with reliable sources like scientific studies and government reports.
- Use Persuasive Language: Employ ethos, pathos, and logos , and use vivid examples to make your points relatable.
- Organize Your Essay: Create a solid persuasive essay outline and ensure a logical flow, with each paragraph focusing on a single point.
- Emphasize Benefits: Highlight how your suggestions can improve public health, safety, or well-being.
- Use Visuals: Incorporate graphs, charts, and statistics to reinforce your arguments.
- Call to Action: End your essay conclusion with a strong call to action, encouraging readers to take a specific step.
- Revise and Edit: Proofread for grammar, spelling, and clarity, ensuring smooth writing flow.
- Seek Feedback: Have someone else review your essay for valuable insights and improvements.
Tough Essay Due? Hire Tough Writers!
Common Topics for a Persuasive Essay on COVID-19
Here are some persuasive essay topics on COVID-19:
- The Importance of Vaccination Mandates for COVID-19 Control
- Balancing Public Health and Personal Freedom During a Pandemic
- The Economic Impact of Lockdowns vs. Public Health Benefits
- The Role of Misinformation in Fueling Vaccine Hesitancy
- Remote Learning vs. In-Person Education: What's Best for Students?
- The Ethics of Vaccine Distribution: Prioritizing Vulnerable Populations
- The Mental Health Crisis Amidst the COVID-19 Pandemic
- The Long-Term Effects of COVID-19 on Healthcare Systems
- Global Cooperation vs. Vaccine Nationalism in Fighting the Pandemic
- The Future of Telemedicine: Expanding Healthcare Access Post-COVID-19
In search of more inspiring topics for your next persuasive essay? Our persuasive essay topics blog has plenty of ideas!
To sum it up,
You’ve explored great sample essays and picked up some useful tips. You now have the tools you need to write a persuasive essay about Covid-19. So don’t let doubts hold you back—start writing!
If you’re feeling stuck or need a bit of extra help, don’t worry! MyPerfectWords.com offers a professional persuasive essay writing service that can assist you. Our experienced essay writers are ready to help you craft a well-structured, insightful paper on Covid-19.
Just place your “ do my essay for me ” request today, and let us take care of the rest!
Frequently Asked Questions
What is a good title for a covid-19 essay.
A good title for a COVID-19 essay should be clear, engaging, and reflective of the essay's content. Examples include:
- "The Impact of COVID-19 on Global Health"
- "How COVID-19 Has Transformed Our Daily Lives"
- "COVID-19: Lessons Learned and Future Implications"
How do I write an informative essay about COVID-19?
To write an informative essay about COVID-19, follow these steps:
- Choose a specific focus: Select a particular aspect of COVID-19, such as its transmission, symptoms, or vaccines.
- Research thoroughly: Gather information from credible sources like scientific journals and official health organizations.
- Organize your content: Structure your essay with an introduction, body paragraphs, and a conclusion.
- Present facts clearly: Use clear, concise language to convey information accurately.
- Include visuals: Use charts or graphs to illustrate data and make your essay more engaging.
How do I write an expository essay about COVID-19?
To write an expository essay about COVID-19, follow these steps:
- Select a clear topic: Focus on a specific question or issue related to COVID-19.
- Conduct thorough research: Use reliable sources to gather information.
- Create an outline: Organize your essay with an introduction, body paragraphs, and a conclusion.
- Explain the topic: Use facts and examples to explain the chosen aspect of COVID-19 in detail.
- Maintain objectivity: Present information in a neutral and unbiased manner.
- Edit and revise: Proofread your essay for clarity, coherence, and accuracy.
Write Essay Within 60 Seconds!

Caleb S. has been providing writing services for over five years and has a Masters degree from Oxford University. He is an expert in his craft and takes great pride in helping students achieve their academic goals. Caleb is a dedicated professional who always puts his clients first.
Struggling With Your Paper?
Get a custom paper written at
With a FREE Turnitin report, and a 100% money-back guarantee
LIMITED TIME ONLY!
Keep reading

OFFER EXPIRES SOON!
REVIEW article
Impact of vaccines; health, economic and social perspectives.

- 1 Department of Zoology, University of Oxford, Oxford, United Kingdom
- 2 Department of Paediatric Infectious Diseases, St George’s University Hospitals NHS Foundation Trust, London, United Kingdom
- 3 Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, United States
In the 20th century, the development, licensing and implementation of vaccines as part of large, systematic immunization programs started to address health inequities that existed globally. However, at the time of writing, access to vaccines that prevent life-threatening infectious diseases remains unequal to all infants, children and adults in the world. This is a problem that many individuals and agencies are working hard to address globally. As clinicians and biomedical scientists we often focus on the health benefits that vaccines provide, in the prevention of ill-health and death from infectious pathogens. Here we discuss the health, economic and social benefits of vaccines that have been identified and studied in recent years, impacting all regions and all age groups. After learning of the emergence of SARS-CoV-2 virus in December 2019, and its potential for global dissemination to cause COVID-19 disease was realized, there was an urgent need to develop vaccines at an unprecedented rate and scale. As we appreciate and quantify the health, economic and social benefits of vaccines and immunization programs to individuals and society, we should endeavor to communicate this to the public and policy makers, for the benefit of endemic, epidemic, and pandemic diseases.
Introduction
“The impact of vaccination on the health of the world’s peoples is hard to exaggerate. With the exception of safe water, no other modality has had such a major effect on mortality reduction and population growth” ( Plotkin and Mortimer, 1988 ).
The development of safe and efficacious vaccination against diseases that cause substantial morbidity and mortality has been one of the foremost scientific advances of the 21st century. Vaccination, along with sanitation and clean drinking water, are public health interventions that are undeniably responsible for improved health outcomes globally. It is estimated that vaccines have prevented 6 million deaths from vaccine-preventable diseases annually ( Ehreth, 2003 ). By 2055, the earth’s population is estimated to reach almost 10 billion ( United Nations Department of Economic and Social Affairs, 2019 ), a feat that in part is due to effective vaccines that prevent disease and prolong life expectancy across all continents. That said, there is still much to be done to ensure the financing, provision, distribution, and administration of vaccines to all populations, in particular those which are difficult to reach, including those skeptical about their protective value and those living in civil disruption. Agencies including the World Health Organization (WHO), United Nations Children’s Fund (UNICEF), Gavi, the Vaccine Alliance, The Bill & Melinda Gates Foundation, and the Coalition for Epidemic Preparedness Initiative (CEPI), with their multiple funding streams have been instrumental in expanding vaccine benefits to all. These importance of these organizations in global co-operation and participation was essential in the setting of the 2019 global pandemic of SARS-CoV-2, in light of the health and economic impact of COVID-19 on societies in high-, middle- and low-income countries. This review will highlight the benefits of vaccinations to society from the perspectives of health, economy, and social fabric ( Figure 1 ), which need to be considered in the overall assessment of impact to ensure that vaccines are prioritized by those making funding decisions.
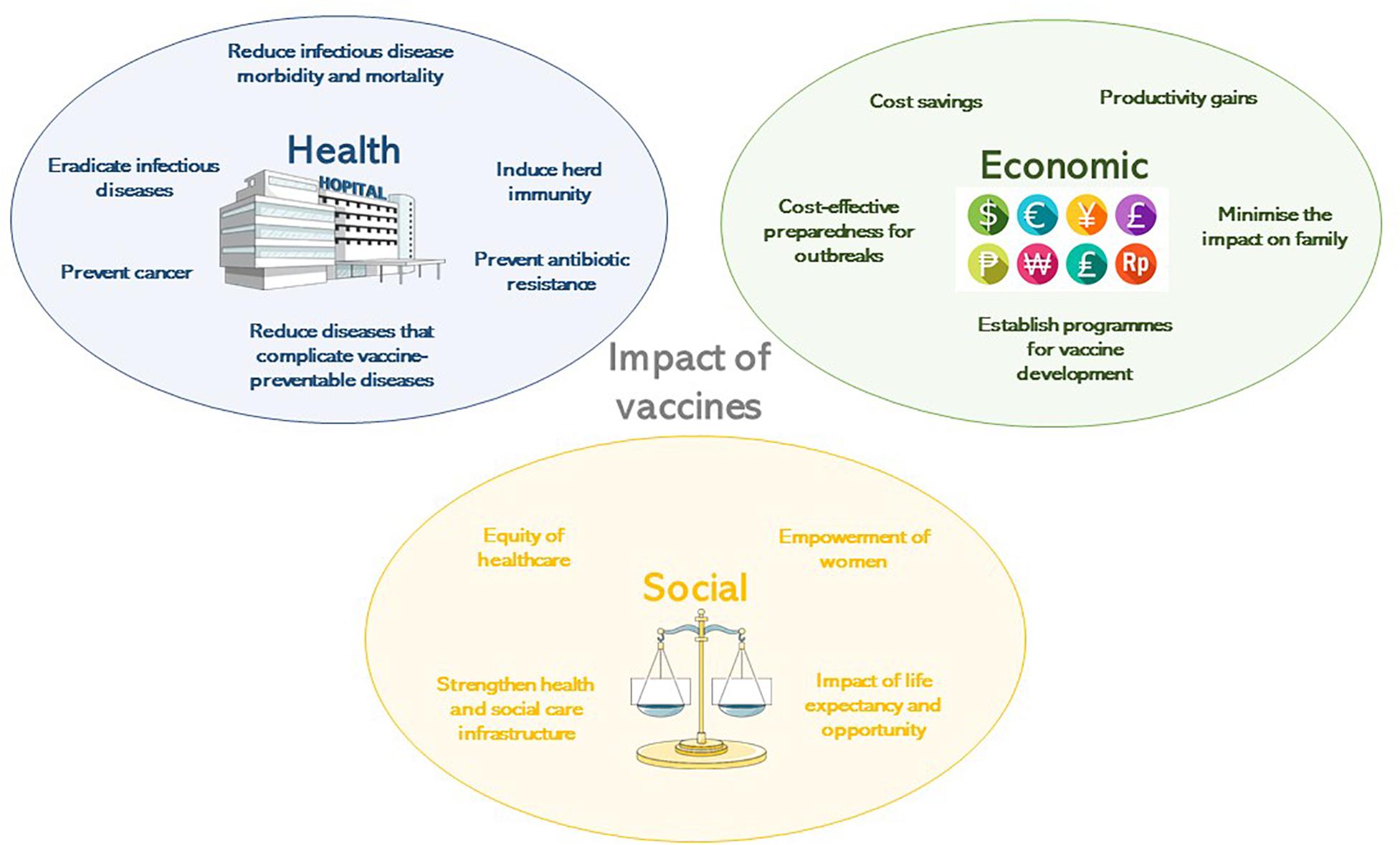
Figure 1. The impact of vaccines according to their health, economic or social benefit.
Brief History of Vaccine Development
Human use of preparations to prevent specific infections have been described since 1500 AD, beginning in China ( Needham, 2000 ) where smallpox was prevented by variolation, which is the introduction of material from scabs into the skin. In 1796 in the United Kingdom, Edward Jenner observed the immunity to smallpox of milkmaids having previously had natural infection with cowpox ( Jenner, 1798 ). He determined that inoculating small amounts of pus from the lesions of cowpox, presumably containing a virus related to vaccinia, into susceptible hosts rendered them immune to smallpox. The vaccine against smallpox was developed in 1798. The next phase of scientific developments involving the manipulation of infectious agents to extract suitable vaccine antigens took almost a century of research. Louis Pasteur’s work with attenuation by oxygen or heat led to live-attenuated chicken cholera, inactivated anthrax and live-attenuated rabies vaccines at the turn of the 20th century ( Pasteur, 1880 , 1881 , 1885 ). Alternative methods of attenuation using serial passage of Mycobacterium bovis led to the live Bacille Calmette-Guerin (BCG) ( Calmette, 1927 ) vaccine, still in use today for the prevention of tuberculosis. Serial passage was also used in the development of yellow fever vaccines ( Theiler and Smith, 1937a ) which are grown in chicken embryo tissues ( Theiler and Smith, 1937b ). Whole cell killed bacterial vaccines were developed when methods to treat and kill bacteria through heat or chemicals were established and whole cell typhoid, cholera and pertussis vaccines resulted at the end of the 19th Century. In 1923, Alexander Glenny and Barbara Hopkins developed methods to inactivate bacterial toxins with formaldehyde, leading to the diphtheria and tetanus toxoid vaccines ( Glenny and Hopkins, 1923 ).
Advances in virus culture in vitro allowed viral pathogens to be studied in greater detail and attenuation methods due to cultivation in artificial conditions led to the live oral polio, measles, rubella, mumps and varicella virus vaccines. In the 1960’s at the Walter Reed Army Institute of Research, vaccines were developed using capsular polysaccharides ( Gold and Artenstein, 1971 ; Artenstein, 1975 ), of encapsulated organisms including meningococci and later pneumococci ( Austrian, 1989 ) and Haemophilus influenzae type b (Hib) ( Anderson et al., 1972 ). To protect against multiple serotype variants of polysaccharide capsules, polyvalent vaccines were developed and later conjugated to carrier proteins to enhance their efficacy in infants in particular by recruiting T-cell mediated help to induce memory B-cells ( Schneerson et al., 1980 ). Vaccines made solely from proteins were rare, with the exception of the toxoid vaccines, but the acellular pertussis vaccine containing five protein antigens, was developed to mitigate the unwanted effects of the whole cell vaccine ( Sato and Sato, 1999 ).
The end of the 20th century marked a revolution in molecular biology and provided insights into microbiology and immunology allowing a greater understanding of pathogen epitopes and host responses to vaccination. Molecular genetics and genome sequencing has enabled the development of vaccines against RNA viruses possessing multiple variants of epitopes, such as the live and inactivated influenza vaccines ( Maassab and DeBorde, 1985 ) and live rotavirus vaccines ( Clark et al., 2006 ). DNA manipulation and excision allowed the use of surface antigen for hepatitis B viral vectors ( Plotkin, 2014 ). The human papilloma virus (HPV) vaccine benefits from enhanced immunogenicity due to the formation of virus-like particles by the L1 antigen of each virus contained in the vaccine ( Kirnbauer et al., 1992 ). Bacterial genome sequencing has provided in depth analysis of meningococcal antigens, to identify potential proteins for meningococcal B vaccines ( Serruto et al., 2012 ).
Vaccine development was tested in 2020 when a novel coronavirus, SARS-CoV-2, emerged from China causing a severe acute respiratory illness, which subsequently spread globally. Within 5 months of the discovery of this virus (7th January 2020) ( Zhu et al., 2020 ) and person-person transmission ( Chan et al., 2020 ), 5,697,334 cases had been identified, with orders of magnitude likely not measured and almost no country escaped the pandemic. Owing to the previous advances in vaccinology, by 8th April 2020, there were 73 vaccine candidates under pre-clinical investigation ( Thanh Le et al., 2020 ). Of these, six were in Phase 1 or 1/2 trials and one was in Phase 2/3 trials by 28th May 2020. The rapidity of this response demonstrated the ability to harness existing technologies including: RNA vaccine platforms (NCT04283461), DNA vaccine platforms (NCT04336410), recombinant vector vaccines (NCT04313127, NCT04324606) and adjuvants. The regulation, manufacturer and distribution of these vaccines will require expedition given the global public health need, from a period of many years to a matter of months. The efficacy and health impact of these vaccines is yet to be established, but if they are effective, then vaccines need to be made available for all global regions affected by SARS-CoV-2. The funding of this endeavor will prove challenging in a global context of national social and economic lockdown and massive government borrowing, but the justification for this provision will be through the multiple benefits to society that will need healthy citizens to rebuild economies in the decades post-COVID-19.
The history of vaccination is not complete without describing the public health intervention that led to the routine use of these vaccines for children globally. The Expanded Program of Immunization (EPI) was founded by WHO in 1974 with the aim of providing routine vaccines to all children by 1990 ( World Health Assembly, 1974 ). In 1977, global policies for immunization against diphtheria, pertussis, tetanus, measles, polio, and tuberculosis were set out. The EPI includes hepatitis B, Hib, and pneumococcal vaccines in many areas and by 2017, 85% of the world’s children (12–23 months of age) received diphtheria, pertussis, tetanus, and measles vaccines ( World Bank, 2019 ).
Health Benefits of Vaccination
Reduction in infectious diseases morbidity and mortality.
The most significant impact of vaccines has been to prevent morbidity and mortality from serious infections that disproportionately affect children. Vaccines are estimated to prevent almost six million deaths/year and to save 386 million life years and 96 million disability-adjusted life years (DALYs) globally ( Ehreth, 2003 ). The traditional measures of vaccine impact include: vaccine efficacy, the direct protection offered to a vaccinated group under optimal conditions e.g., trial settings; or vaccine effectiveness, the direct and indirect effect of vaccines on the population in a real-life setting ( Wilder-Smith et al., 2017 ). Providing a numerical measure of vaccine impact therefore involves estimating the extent of morbidity and mortality prevented. In the United States in 2009, amongst an annual birth cohort vaccinated against 13 diseases it was estimated that nearly 20 million cases of disease and ∼42,000 deaths were prevented ( Zhou et al., 2009 ). Infectious diseases that accounted for major mortality and morbidity in the early 20th century in the United States all showed over a 90% decline in incidence by 2017 from the pre-vaccine peak incidence ( Roush and Murphy, 2007 ), due to high vaccine uptake of over 90% for the DTaP (diphtheria, tetanus, and acellular pertussis), MMR (measles, mumps, and rubella) and polio vaccines ( World Health Organisation, 2019a ; Table 1 ). A similar pattern of infectious diseases reduction was seen across other high-income countries, demonstrating the efficacy of vaccines when available and accessible.
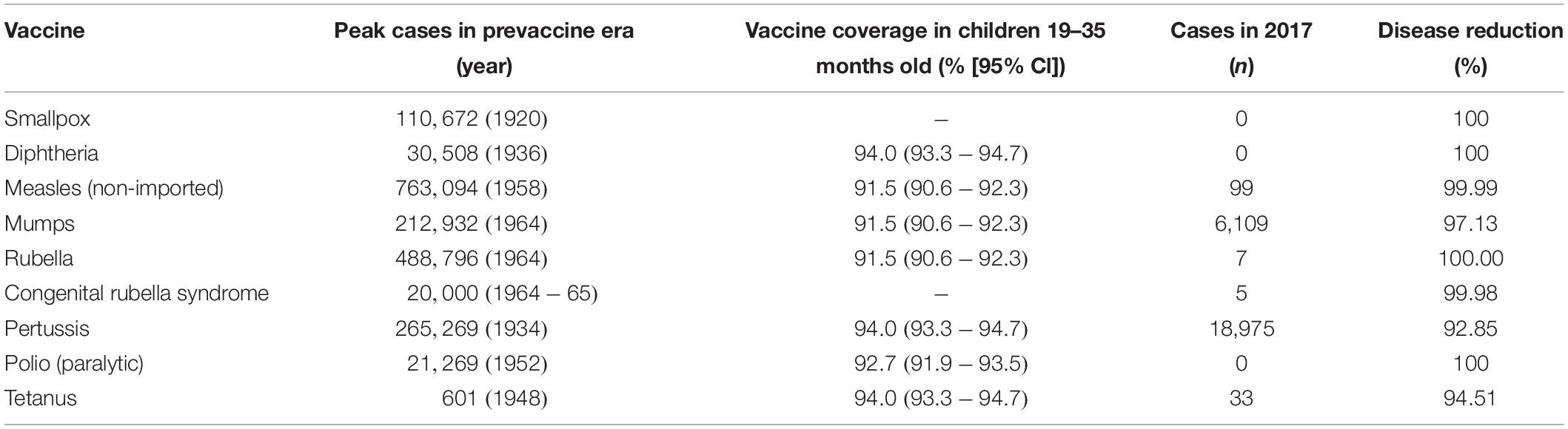
Table 1. Vaccine impact in United States comparing the incidence of diseases prior to the implementation of vaccine ( Roush and Murphy, 2007 ), described as the pre-vaccine era and the vaccine coverage ( Hill et al., 2017 ) and disease incidence ( Centers for Disease Control and Prevention, 2017 ) in 2017, as reported by the Centers for Disease Control and Prevention.
Globally, the provision of vaccines is more challenging in many low- and middle- income countries (LMIC), as evidenced by the failure to make the EPI vaccines available to every child by 1990, irrespective of setting ( Keja et al., 1988 ). Central to this is limited financial resources, but other barriers to vaccine introduction include: underappreciation of the value of vaccines locally/regionally though insufficient relevant data on disease burden, vaccine efficacy, or cost-effectiveness; inadequate healthcare infrastructure for vaccine handling, storage, programmatic management, and disease surveillance; and lack of global, regional or local policy-making and leadership ( Munira and Fritzen, 2007 ; Hajjeh, 2011 ). In 2018, the global uptake of three doses of DTaP reached 86% which corresponded to 116,300,000 infants ( World Health Organisation, 2019a ). The vaccine coverage is, however, variable between low-, middle- and high-income countries because of a combination of economic and political circumstances as well as variable access to non-governmental support from Gavi, the Vaccine Alliance ( Turner et al., 2018 ; Figure 2 ). Nevertheless, there has been a decrease in the global burden of diseases caused by vaccine-preventable pathogens ( Figure 3 ) enabling healthier lives for many millions of children. A further benefit following vaccination, is the evidence that although vaccines may not always prevent an infection, for example VZV or pertussis, a milder disease course may follow ( Andre et al., 2008 ; Bonanni et al., 2015 ).
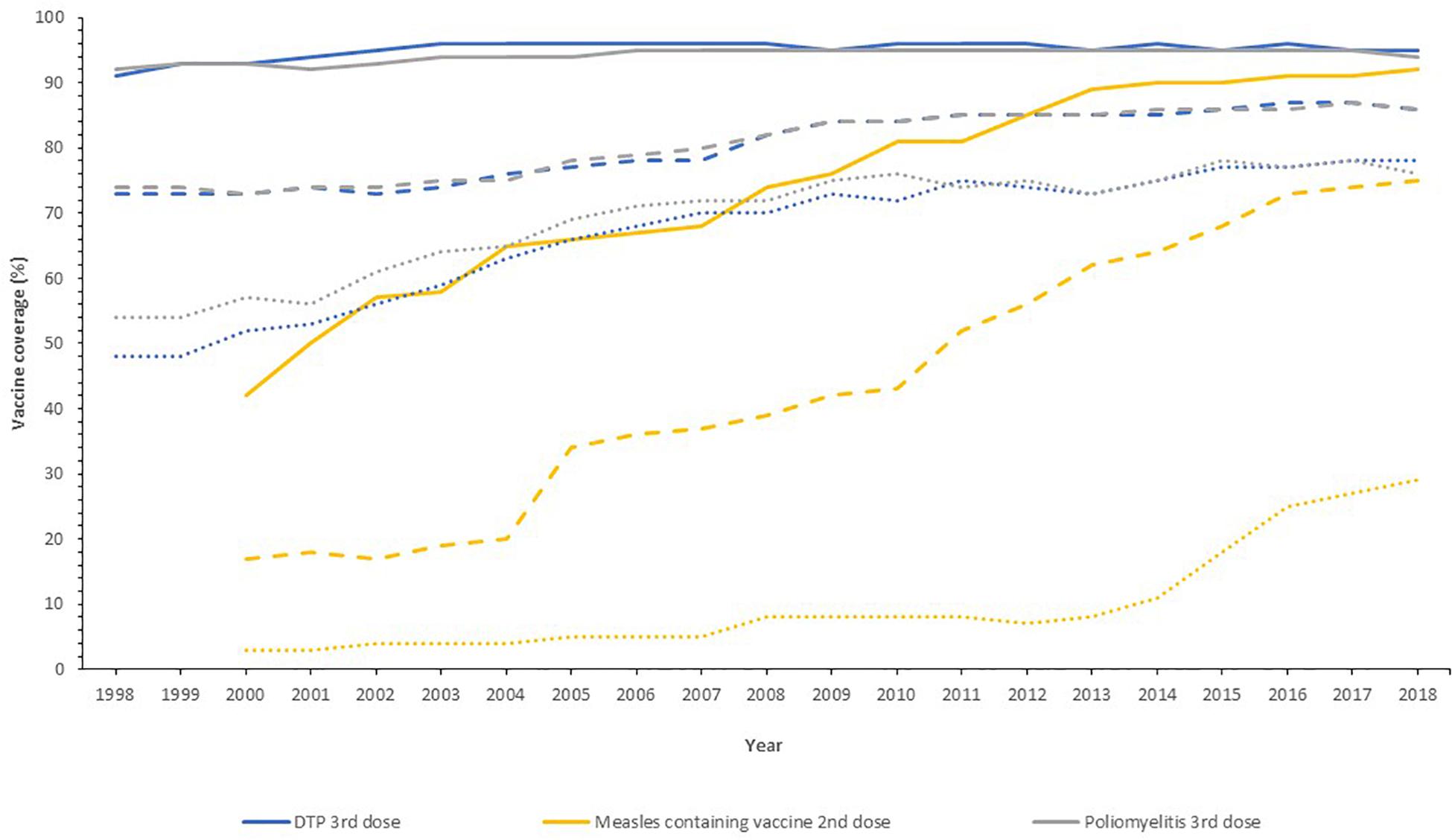
Figure 2. Vaccine uptake across different regions defined by economic status by the World Bank into high- (solid line), middle- (dashed line), and low-income countries (dotted line) for the past 20 years. Data from the World Health Organization and UNICEF dataset “Coverage Estimates Series” ( World Health Organization [WHO] and United Nations Children’s Fund [UNICEF], 2019 ).
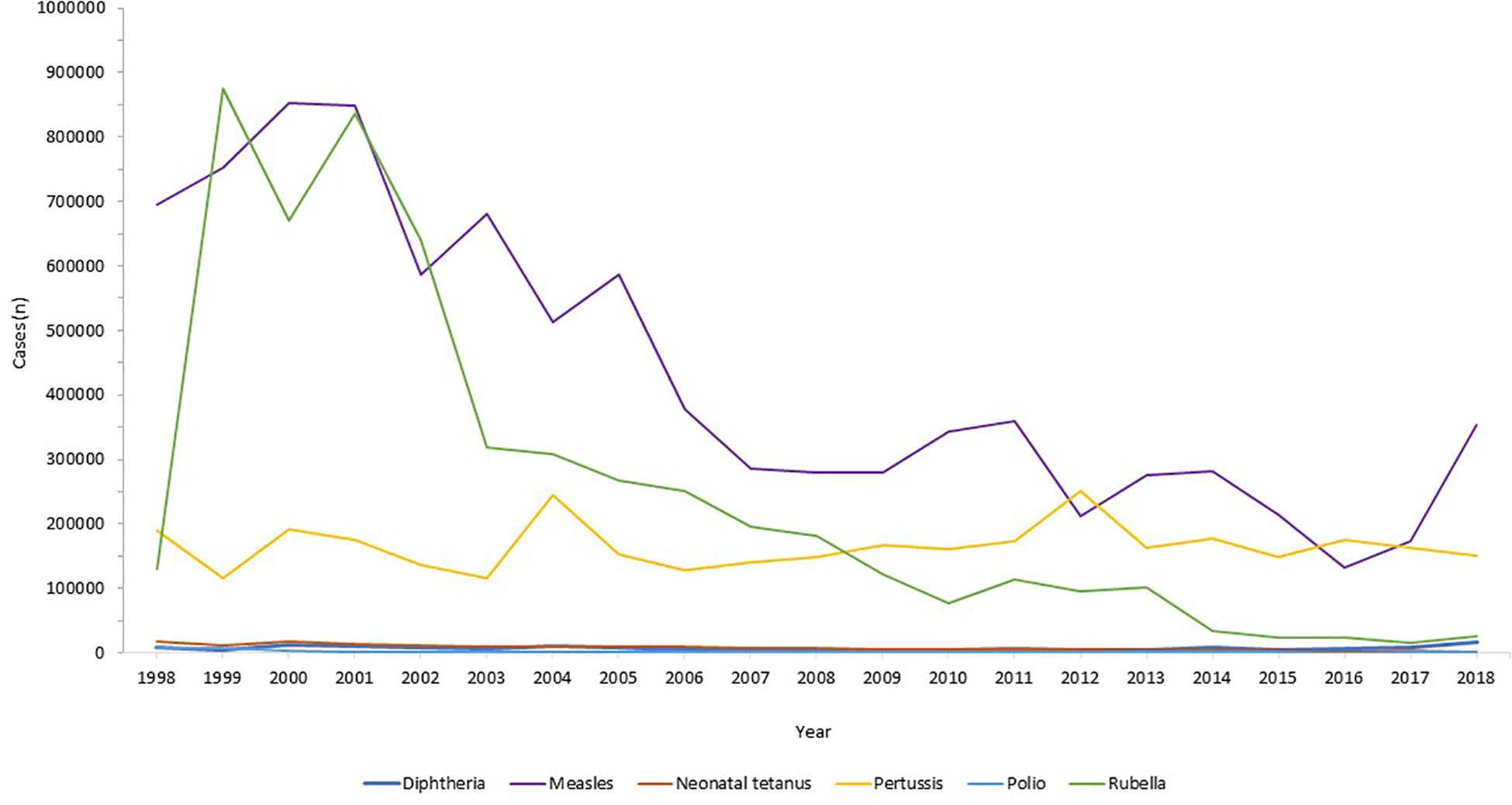
Figure 3. Reduction in infectious diseases globally. Across all world regions, data from the WHO, for the last 20 years showing the control of diphtheria and tetanus and the decline in rubella and congenital rubella syndrome (data not shown). Data from the World Health Organization dataset “Reported cases of vaccine-preventable diseases” ( World Health Organisation, 2019c ).
Eradication of Infectious Diseases
Global disease eradication can be achieved for pathogens that are restricted to human reservoirs. For eradication of infectious diseases, high levels of population immunity are required globally, to ensure no ongoing transmission in our well-connected world ( Andre et al., 2008 ). Furthermore, surveillance systems must be in place to monitor the decline in disease, with accurate and reliable diagnostic testing to monitor ongoing cases. At the time of writing, the only infectious disease that has been eradicated in humans by vaccination is smallpox. This disease had afflicted humans for millenia, with the earliest evidence found in Egyptian mummies from 1000 BC ( Geddes, 2006 ). Jenner’s successful development of the smallpox vaccine using vaccinia virus ( Jenner, 1798 ) led to the ultimate eradication of the disease through ring vaccination as announced by the World Health Assembly in 1980 ( Strassburg, 1982 ), which was an historic public health achievement. The second example of eradication was of the rinderpest virus in livestock, an infection that indirectly led to human loss of life through loss of agriculture leading to humanitarian crises through famine and poverty. Rinderpest virus infects cattle, buffalo and numerous other domestic species, with widespread disease affecting large parts of Africa and Europe in the 19th century ( Roeder et al., 2013 ). The Plowright tissue culture rinderpest vaccine, developed during the 1950s, was used for mass vaccination campaigns, alongside other public health measures, leading to eradication in 2011 ( Morens et al., 2011 ).
The next infection targeted for eradication is wild polio virus. This devastating paralytic disease routinely afflicted children and adults in both industrialized and developing settings, prior to the development of vaccines. Two polio vaccines, the inactivated polio vaccine (IPV) and the live-attenuated oral polio vaccine (OPV) became available in 1955 and 1963, respectively ( Plotkin, 2014 ), both able to protect against all three wild types of polio virus. Both vaccines have been used globally, with live-attenuated OPV much cheaper and easier to administer but carrying the risk of causing circulating vaccine-derived poliovirus (cVDPV) owing to back-mutation and re-acquisition of neurovirulence. Hence, due to its safety IPV was preferred in industrialized regions and those where the polio incidence was low. In 1998, the Global Polio Eradication Initiative, the largest public-private partnership led by national governments in partnership with the WHO, Rotary International, United States Centers for Disease Control and Prevention (CDC), and UNICEF was launched with the aim of global polio eradication by 2000. Although this target was not met due to lack of funding, political will, and competing health initiatives, there was a 99% reduction in polio incidence by 2000 ( Lien and Heymann, 2013 ). By 2003, there were only six endemic countries with new cases: Egypt, Niger, India, Nigeria, Afghanistan, and Pakistan, of which only the latter four had new cases by 2005. Eradication in India was problematic due to the high birth rates and poor sanitation amongst densely populated regions with marginalized communities and high population mobility ( Thacker et al., 2016 ). India was declared polio free in 2014. Wild polio virus type 2 was eradicated in 2015, the last case of wild type 3 was in 2012 and eradication announced in 2019, with wild type 1 virus remaining in two countries, Pakistan and Afghanistan ( World Health Organisation, 2019b ). In 2019, Nigeria was declared 3 years free of wild polio, the last country in Africa to declare any cases. In the first 6 months of 2020, there were 51 and 17 cases of wild type 1 polio reported in Pakistan and Afghanistan respectively ( Global Polio Eradication Initiative, 2019 ). Ongoing programs to roll out universal vaccination in both countries remain hindered by armed conflict, political instability, remote communities and underdeveloped infrastructure. The risk of the OPV recipients developing cVDPV disease, with transmission through the faeco-oral route to cause outbreaks of vaccine-derived paralytic poliomyelitis remains a concerning obstacle in the eradication process, requiring intensive surveillance.
Herd Immunity
The overriding health benefit perceived by most vaccine recipients is their personal, direct, protection. The added value of vaccination, on a population level, is the potential to generate herd immunity. Where a sufficiently high proportion of the population are vaccinated, transmission of the infecting agent is halted thereby protecting the unvaccinated, who may be those too young, too vulnerable, or too immunosuppressed to receive vaccines. Highly successful vaccination programs have been in place as part of the routine EPI, against encapsulated bacteria that are carried asymptomatically in the oropharynx but that can invade and cause septicemia and meningitis in all age groups. Vaccines against Neisseria meningitidis ( Gold and Artenstein, 1971 ), Streptococcus pneumoniae ( Austrian, 1989 ), and Hib ( Anderson et al., 1972 ) were developed in the 1960s, 1970s, and 1980s, respectively, using their polysaccharide capsules as vaccine antigens, which successfully induced protective immunity (direct protection). Conjugation of these polysaccharides to carrier proteins in the 1990s improved their efficacy by not only ensuring a T cell response and immune memory, but by reducing acquisition of pharyngeal carriage of these organisms, thus providing indirect protection and thereby preventing ongoing transmission ( Pollard et al., 2009 ). This was first observed in national carriage studies in the United Kingdom in 1999–2001 during a mass vaccination campaign against serogroup C N. meningitidis ( Maiden et al., 2008 ) and was a major contributing factor to the declining disease thereafter.
Herd (population) immunity requires high levels of vaccine uptake, to limit the number of unvaccinated people and the opportunity for pathogen transmission between them. The proportion of a given population required to induce herd immunity through vaccination is lower for the bacterial infections and conjugate polysaccharide vaccines, as their basic reproductive number (R 0 ) is lower than viral infections like measles, varicella or polio ( Table 2 ). Measles virus can cause devastating disease ranging from acute presentations with pneumonia or encephalitis, to immune amnesia and long-term complications such as subacute sclerosing panencephalitis ( Mina et al., 2015 , 2019 ; Moss, 2017 ; Petrova et al., 2019 ). The live-attenuated measles vaccine is highly efficacious and the first dose is recommended at 9–12 months of age. To protect those who cannot receive live vaccines (younger infants, pregnant women, the immunosuppressed) from acquiring measles in the community, at least 93–95% of the population is required to be vaccinated with two doses in order to interrupt measles virus transmission. In many countries in Europe and in the United States, this level of vaccination uptake is falling ( Wise, 2018 ), due to a combination of reduced accessibility to health services and vaccine misinformation. As a result, some countries, including the United Kingdom and United States, where elimination of measles had been declared have had a resurgence of disease ( Wise, 2019 ). For high-risk individuals who are unable to be vaccinated, herd immunity represents a life-saving protection strategy against many infections. An alternative strategy, cocooning, has been employed with limited success for pertussis and influenza ( Grizas et al., 2012 ), where their close/household contacts are vaccinated to prevent transmission.
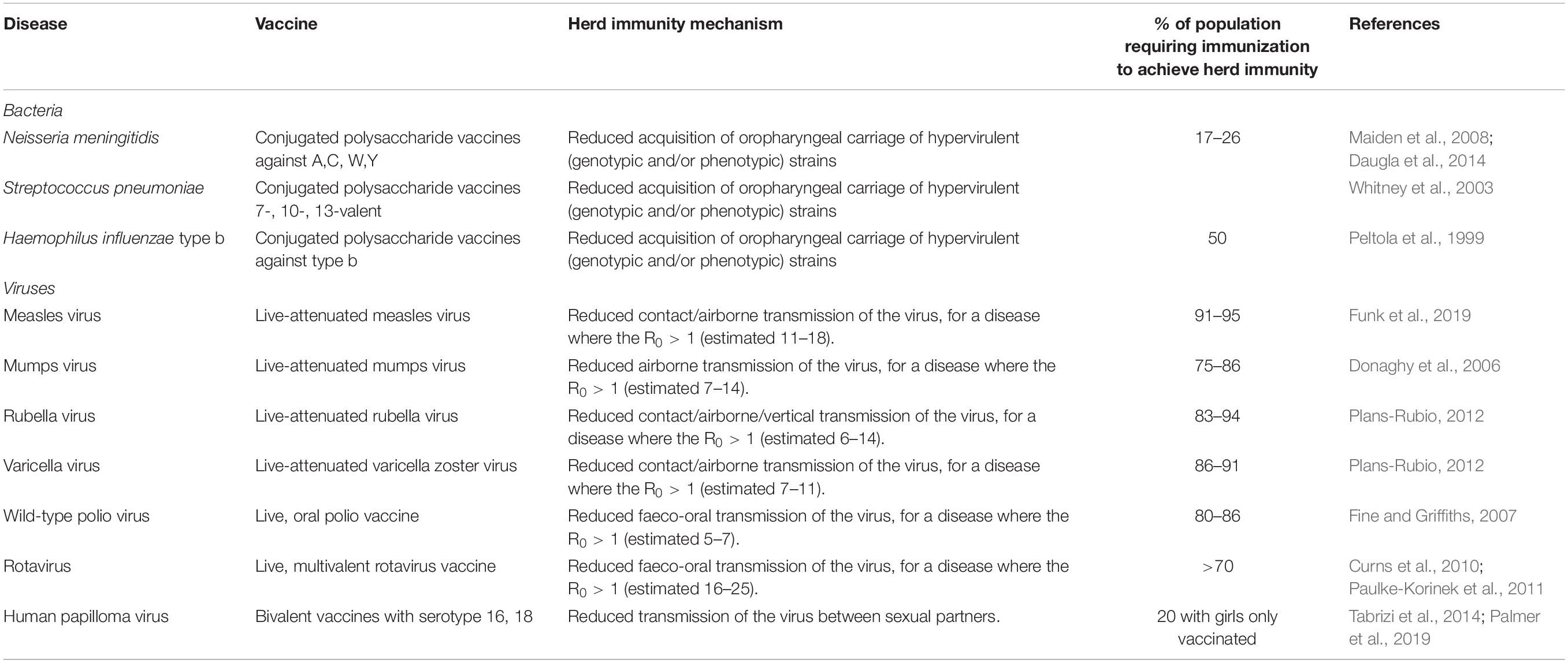
Table 2. Vaccines with the potential to induce herd immunity, with the infectious agent, vaccine type, and thresholds of population vaccination needed for herd immunity ( Peltola et al., 1999 ; Whitney et al., 2003 ; Donaghy et al., 2006 ; Fine and Griffiths, 2007 ; Maiden et al., 2008 ; Curns et al., 2010 ; Paulke-Korinek et al., 2011 ; Plans-Rubio, 2012 ; Daugla et al., 2014 ; Tabrizi et al., 2014 ; Funk et al., 2019 ; Palmer et al., 2019 ).
Herd immunity has been observed for gastrointestinal infections with vaccines against cholera (oral cholera vaccine) and rotavirus (oral rotavirus vaccines). Early adopters of rotavirus vaccines included the United States (2006) and Austria (2007) where there were dramatic reductions in disease observed in the vaccinated infant cohort, and also in the older age groups of children and adults ( Curns et al., 2010 ; Paulke-Korinek et al., 2011 ), suggesting that the reduction in disease and shedding of virus in the stool stopped transmission to healthy household contacts. For the OPV, herd protection may also be induced through vaccine virus shedding and spread to unvaccinated people ( Fine and Griffiths, 2007 ).
Reduction in Secondary Infections That Complicate Vaccine-Preventable Diseases
Vaccines can prevent diseases beyond the specific infection they are designed to target. Infections with pathogens, in particular viruses, can predispose to the acquisition of other bacterial infections. For example, influenza virus infection, both seasonal and pandemic, is frequently complicated by bacterial pneumonia and acute otitis media (OM), and infrequently Aspergillus pneumonia/pneumonitis. During the influenza pandemic of 1918–19, secondary bacterial bronchopneumonia with S. pneumoniae, Streptococcus pyogenes , H. influenzae , and Staphylococcus aureus identified at autopsy, likely contributed to the excess mortality observed amongst healthy children and adults ( Morens and Fauci, 2007 ). Influenza vaccinations can be beneficial in preventing these complications and also morbidity including acute OM in children; a systematic review demonstrated influenza vaccine efficacy against OM of 51% (21–70%) ( Manzoli et al., 2007 ). Further, there is evidence that inactivated influenza vaccines administered to pregnant women can reduce the hospital admission with acute respiratory illnesses in their infants up to 6 months of age ( Regan et al., 2016 ). Amongst pregnant, HIV-negative women in South Africa, infants (<3 months) were protected against hospitalization with all-cause lower respiratory tract infections with a vaccine efficacy of 43% ( p = 0.05), including primary viral and secondary bacterial causes ( Nunes et al., 2017 ). Additionally, in children pneumococcal conjugate vaccines were observed to reduce the incidence of influenza-associated hospital admissions in United States ( Simonsen et al., 2011 ), Spain ( Dominguez et al., 2013 ), and South Africa ( Madhi et al., 2004 ; Abadom et al., 2016 ), through the prevention of secondary bacterial infections following primary influenza infection.
The introduction of the live-attenuated measles vaccine in the 1970s was observed to reduce both measles and non-measles mortality in children ( Aaby et al., 2003 ). Measles causes severe pneumonia, encephalitis, and the long-term sequel of subacute sclerosing panencephalitis ( Moss, 2017 ), but the decline in mortality was not limited to preventing these alone ( Aaby et al., 2003 ). Mathematical modeling of vaccination and immunological research demonstrated that measles causes an immunological amnesia, eliminating B cell populations and thus immune memory, leaving measles survivors susceptible to all the infective agents they had previously developed immunity against; it is estimated to take 3 years for immune recovery to occur ( Mina et al., 2015 ).
Prevention of Cancer
Historically, vaccines were developed against very severe infections with major morbidity and mortality from acute disease. As non-communicable diseases, including cancer, become the most frequent causes of death in industrialized countries and some developing countries, vaccines are being used to prevent these too, when the infectious agents are involved in carcinogenesis. Hepatitis B prevalence is high in regions of East Asia, sub-Saharan Africa, and the Pacific Islands. Chronic hepatitis B infection can lead to liver cirrhosis and hepatocellular carcinoma ( Bogler et al., 2018 ). Vertical transmission of hepatitis B is problematic as 70–90% of babies born to HbsAg and HbeAg positive mothers will become infected without prophylaxis administered to babies; with ∼90% of infants developing chronic hepatitis ( Borgia et al., 2012 ; Gentile and Borgia, 2014 ). The chronic hepatitis B carriage status of mothers is routinely checked at the start of pregnancy, in order to assess the need to vaccinate the infant after birth. The use of both hepatitis B vaccine, containing hepatitis B surface antigen, and immunoglobulin containing hepatitis B antibody can be used to minimize vertical transmission, with evidence from a 20-year-long study in Thailand demonstrating 100% prevention of transmission ( Poovorawan et al., 2011 ).
The sexually transmitted HPV is responsible for genital tract and oropharyngeal infections as a precursor to causing oncological disease affecting the cervix, vagina, vulva, penis, anal tract, and pharynx in both men and women. Cervical cancer is the fourth most common cancer globally, with 528,000 new cases annually and peak incidence in young women aged 25–34 years ( Ferlay et al., 2012 ). The HPV serotypes 16 and 18 carry a high-risk for cervical cancer ( Wang et al., 2018 ) and vaccination against these specific serotypes has been available since 2006 through bivalent (16 and 18), quadrivalent (6, 11, 16, and 18), and nonavalent (6, 11, 16, 18, 31, 33, 45, 52, 58) vaccines, which are now available to individuals from the age of 9 years ( Gupta et al., 2017 ). A vaccination program started in the United Kingdom in 2008, and at the time of writing over 10.5 million doses had been given to girls ( Public Health England, 2018 ), with the aim of preventing primary infection with HPV. The vaccine coverage was 83.8% for 13–14 year old girls in England in 2017/18 ( Public Health England, 2019 ). In July 2018, the vaccine was approved for use in boys ( Public Health England, 2019 ). After a decade of use, there has been an observed decline in the genital infections caused by serotypes 16 and 18 ( Public Health England, 2018 ), with further time needed to observe the fall in cervical cancer incidence. However, the incidence of pre-invasive cervical diseases has been reduced by 79–89% in Scottish women over 20 who were vaccinated with bivalent HPV vaccine when aged 12–13 years, with evidence of herd protection ( Palmer et al., 2019 ), offering a promising outlook for the reduction of cervical cancer in the future. An additional benefit of HPV vaccines, is their impact on neonatal morbidity and mortality, through the reduction in surgical treatment of cervical neoplasias, and the related preterm births and complications ( Soergel et al., 2012 ).
Preventing Antibiotic Resistance
The rise in antimicrobial resistance (AMR) is a universal threat. The use of antibiotics in humans, exposes the bacteria that reside in our microbiota to selection pressures resulting in the development of AMR. As the bacteria constituting the host microbiota are frequently responsible for invasive diseases such as: meningitis, pneumonia, urinary tract, or abdominal infections, the risk of developing infections that are difficult or eventually impossible to treat is fast becoming a reality ( Brinkac et al., 2017 ). In regions where resistant pathogens are circulating at high frequency, such as India or regions of Europe ( Logan and Weinstein, 2017 ), patients will be faced with choosing between having elective surgical procedures or chemotherapy for malignancy, and the risk of acquiring potentially untreatable, multi-drug resistant bacterial infections ( Liu et al., 2016 ). Vaccination is crucial in mitigating this risk, by preventing people from developing viral and bacterial infections in the first instance, and therefore reducing the antibiotic burden to which their microbiota are exposed. The development of AMR in bacteria is a cumulative process with frequent, repeated exposure to broad spectrum antibiotics as a major driver. Children and the elderly who are at particular risk of infection can benefit from vaccines against common primary and secondary infections such as: pneumonia (prevented by PCV, PPSV, influenza, and measles vaccines), OM (PCV, Hib, and measles vaccines), cellulitis secondary to VZV (VZV vaccine), and typhoid fever (typhoid vaccine) which alleviates the need for antibiotics being prescribed or bought ( Kyaw et al., 2006 ; Palmu et al., 2014 ). The extent to which vaccination contributes to antimicrobial stewardship was highlighted by its inclusion in vaccine cost-effectiveness analyses as part of national United Kingdom policy ( Bonanni et al., 2015 ).
Economic Benefits
Cost savings.
Vaccines are highly beneficial on a population level and also cost-effective ( Shearley, 1999 ) in comparison to other public health interventions ( Bloom et al., 2005 ). Government departments are required to perform systematic economic analyses of vaccines and vaccine programs to justify their purchase in view of pressure on public and private finances globally, this was exacerbated by the 2008 financial crash. A vaccination program has clear direct costs including: vaccine purchase, infrastructure to run the program and maintain the cold chain, and healthcare/administration personnel. Governments, sometimes supported by charities and non-governmental organizations, invest in these with the intention of improving health. The reduction in morbidity and mortality associated with successful vaccine programs, through a combination of direct and indirect protection, has led to reduced incidence of diseases and their associated treatments and healthcare costs ( Deogaonkar et al., 2012 ). This potentially leads to economic growth, with less money spent owing to the costs averted through fewer medical tests, procedures, treatments and less time off work by patients/parents. Additionally, the use of combination vaccines e.g., DTaP/IPV/Hib/HepB provides protection against an increased number of diseases, with no additional infrastructure costs i.e. the same number of injections per child within existing immunization programs.
The cost-effectiveness analyses of vaccination programs demonstrate that they are overwhelmingly worth the investment, with most programs costing less than $50 per life gained, orders of magnitude less than prevention of diseases like hypertension ( Ehreth, 2003 ; Bloom et al., 2005 ). The returns on investment in vaccines, given their increasing provision through Gavi, have been estimated at 12–18% ( Bloom et al., 2005 ), but this is likely an underestimate. The monetary advantages of vaccination programs are important both to industrialized nations, such as the United States which obtains a net economic benefit of $69 billion, but also in 94 LMIC where investment of $34 billion, resulted in savings of $586 billion from the direct illness costs ( Ozawa et al., 2016 ; Orenstein and Ahmed, 2017 ). The net economic impact of eradication of disease has been estimated for both smallpox and polio. For smallpox, the eradication costs were over 100 million USD, but there are cost savings of 1.35 billion USD annually, with elimination of polio estimated to save 1.5 billion USD annually ( Barrett, 2004 ; Bloom et al., 2005 ). A less well-considered economic saving, not captured in cost-effectiveness or cost-benefit analyses, is from the prevention of long-term morbidity following acute infections ( Bloom et al., 2005 ), for example hearing impairment following pneumococcal meningitis or limb amputation following meningococcal disease, along with broader productivity gains ( Deogaonkar et al., 2012 ), which could have a major impact on LMIC adoption of vaccine programs.
Productivity Gains
The relationship between health and the economy is bidirectional, whereby economic growth enables funding in investments that improve health; and a healthy population contributes to and enhances an economy. These benefits of vaccinations and other public health interventions including sanitation, clean water, and antibiotics, are important for social as well as economic reasons. It has been suggested that the economic impact of vaccines should be considered more broadly than just the averted healthcare costs from prevented illness episodes and associated carer costs ( Deogaonkar et al., 2012 ; Barnighausen et al., 2014 ; Bonanni et al., 2015 ; Gessner et al., 2017 ; Wilder-Smith et al., 2017 ). Bärnighausen et al. (2011) , set out a framework to consider productivity gains measured by: outcome and behavior; community health and economic externalities; risk reduction; and health gains. Healthy children demonstrate improved educational attainment at school through better attendance and better cognitive performance ( Barham and Calimeria, 2008 ; Bloom et al., 2011 ; Deogaonkar et al., 2012 ). The impact of hearing loss from mumps, rubella or pneumococcal infections, or visual impairment from measles may require specific educational support, whereas the cognitive deficits from those childhood infections may require substantial remedial input. As more children survive to adulthood, a larger adult workforce is available, who when healthy can work for longer and more productively both physically and mentally ( Bloom and Canning, 2000 ; Bloom et al., 2005 ); though to date this has been observed largely following other health improvements, not vaccination specifically ( Jit et al., 2015 ). As a result of vaccination healthy and economically successful populations have lower fertility rates and smaller families ( Sah, 1991 ; Andre et al., 2008 ). With improved health and therefore life expectancy, there is a wider effect on families who may choose to invest more money in their future, for example to enhance their education or through savings ( Jit et al., 2015 ). Overall, vaccine programs should be viewed as an investment in human capital, providing enduring impact on economies worldwide.
Minimizing the Impact on Family
The economic impact of adult illness is evident from loss of productivity and pay for the duration of the illness and recovery period. The impact of childhood illness falls primarily on their adult carers, generally parents. In most industrialized regions, two-parent families are reliant on both parents undertaking at least part-time or full-time work. Therefore, when a child is unwell with childhood illnesses, which may or may not necessitate admission to hospital, the parent will invariably have to forego their paid employment to care for the child. In seven European countries one parent or carer required time off work in 39–91% of rotavirus gastroenteritis cases ( Van der Wielen et al., 2010 ). This loss of productivity in the parental workforce tends to disproportionately affect women, but loss of either parental attendance at work reduces overall employer productivity and in the short-term is rarely replaced. This argument was made for the impact of chicken pox on children, whereby the exclusion from school mandates parental caring at home for a period until the lesions are crusted over. VZV vaccines are estimated to have had a similar impact as rotavirus vaccine in United States studies ( Lieu et al., 1994 ). In many regions, mothers are still the primary carers, spending their days at home caring for children and maintaining the household; in these settings, the impact on this unpaid work is harder to determine.
It is of paramount importance to quantify and include productivity gains and the wider effects in analyses of impact for vaccines with only moderate efficacy, as calculated using traditional metrics. Vaccines such as the RTS,S/AS01 malaria vaccine, CYD-TDV dengue vaccine and rotavirus vaccine used in LMIC all have limited ability to broadly protect populations over a long duration but the public health benefits were important in vaccine implementation decisions in those countries ( Wilder-Smith et al., 2017 ). This suggests a paradigm for alternative regulatory requirements with a focus on public health outcomes ( Gessner et al., 2017 ).
Cost-Effective Preparedness for Outbreaks
As human populations grow and their use of the finite land resources increases, we are in increasingly close association with other living creatures, voluntarily or involuntarily. This interaction with natural reservoirs of potential infectious diseases increases the risk of zoonotic transmission of new infectious pathogens e.g., SARS, MERS-CoV, or known infectious pathogens with increased virulence e.g., influenza. Emerging infectious diseases disproportionately affect developing regions, where health infrastructure and surveillance are likely to be less well-established and robust. There were 1,307 epidemics of infectious diseases between 2011 and 2017, which cumulatively cost $60 billion annually to manage ( GHRF Commission, 2016 ). The unpredictability of outbreaks was highlighted by the Ebola epidemic in Western African countries of Liberia, Sierra Leone, and Guinea in 2014, which occurred in a period when public health was supposedly at its most advanced in recent history. However, a catalog of areas including: outbreak planning infrastructure; disease surveillance; local health services; escalation to international agencies were found to be lacking ( GHRF Commission, 2016 ). Although the WHO received criticism for its lack of escalation, in reality the global and interconnected infrastructure to prevent such epidemics taking lives and devastating societies is insufficient at the present time. The Zika virus epidemic in Latin America in 2015, first observed through an unexpectedly high incidence of microcephaly amongst newborns in Brazil’s northern regions ( Heukelbach et al., 2016 ), provide another example of how epidemics can have lasting impact, with the virus causing significant neurological damage to surviving infants ( Russo et al., 2017 ). The SARS-CoV-2 pandemic which began in 2019, was, at the time of writing, the largest infectious disease pandemic since the influenza pandemic of 1918/9. This global public health crisis highlighted stark societal inequalities persistent in many high-, middle- and low-income countries with direct and indirect impact on health outcomes from this infection. The cost-effectiveness of a vaccine in this setting was unquestionable, with economies and societies shut down for months in early 2020 and likely again in future. As it is not feasible or practical to be able to predict the location or nature of the next emerging threat, investment of an estimated $4.5 billion/year in healthcare systems could help speed up responses to infectious epidemics by prompt identification of the agent and effective control measures to limit the spread and consequences of disease ( GHRF Commission, 2016 ). The importance of this planning within the political landscape and the ongoing threat that infectious disease pose, may be appreciated more widely after 2020.
Establishing Programs for Vaccine Development
One effective infection control method is the use of vaccines in the course of an epidemic to halt transmission and to induce immunity to those as yet unaffected. The cost of vaccine development is a major challenge as there is little incentive for industry to invest in the design, testing and manufacture of vaccines that may never be needed, have a limited market, and, as previously eluded to, may be required in LMIC which cannot afford the upfront costs as an epidemic unfolds. The estimated costs for funding the development of infectious diseases vaccines for epidemics through phase 2a clinical trials are a minimum of $2.8-3.7 billion ( Gouglas et al., 2018 ). The CEPI alliance was established at the Davos World Economic Forum in 2017 as a global partnership between public, private and philanthropic organizations. In response to the conclusion that “a coordinated, international, and intergovernmental plan was needed to develop and deploy new vaccines to prevent future epidemics,” CEPI have identified the most important known global infectious threats and invested in the development of vaccines, stockpiling, and policies to allow equitable access to these ( Plotkin, 2017 ). Further, the establishment of research and development infrastructure pipelines will allow production of suitable vaccine candidates within 16 weeks of identification of a new pathogen antigen. The broader aims including: improving global epidemic responses; capacity building; and global regulation of outbreak management strategies are also within the remit of CEPI’s work. It is these types of preparedness plans that assisted vaccine development and global health collaborations to address the COVID-19 pandemic, though many regions of high-, middle-, and low-income countries alike were slow or resistant to pre-empt and prepare for this type of infectious disease threat.
Social Benefits
Equity of healthcare.
As a result of the combined effects of poverty, malnutrition, poor hygiene and sanitation, overcrowding, discrimination and poorer access to health-care, the underprivileged in society are disproportionately afflicted by infectious diseases. Over the 20th century, it has become a moral standpoint and a human right for every individual to be provided with access to safe vaccines. The provision of vaccination as part of the EPI on a national and international scale ( World Health Assembly, 1974 ) acted as a great leveler to start reducing the impact of infectious diseases to all, regardless of other disadvantages. Over the 15 years of the EPI, the vaccine coverage in developing countries increased from 5% to ∼80% ( Levine and Robins-Browne, 2009 ). The EPI was revolutionary for its time, an ambitious public health program that aimed to improve children’s life chances despite the country and situation in which they were born. The administration of vaccines by UNICEF was deemed so important that there have been at least seven ceasefires in civil conflicts to allow this to happen ( Hotez, 2001 ).
The impact of vaccines on the inequity of those living in poverty is marked. A study of over 16,000 children during the phased introduction of the measles vaccine in Bangladesh in 1982, demonstrated improved health outcome equity when measured by under-5 mortality ( Bishai et al., 2003 ). Further, modeling of the impact of the rotavirus vaccine in India across social strata, which are closely aligned to wealth, suggested that the vaccine program would provide the poor with both health and financial benefits ( Verguet et al., 2013 ). Including such equity impact in the health economic modeling of vaccines would allow policy decisions to be targeted to the most vulnerable in society ( Riumallo-Herl et al., 2018 ). Additional cost-effective benefits observed after the implementation of combined public health initiatives ( Deogaonkar et al., 2012 ; Gessner et al., 2017 ) include provision of vaccines, facilitation of healthcare, reduction of indoor air pollution and improvement of nutrition to prevent childhood pneumonia ( Niessen et al., 2009 ).
Strengthening Health and Social Care Infrastructure
To provide the EPI universally to infants and children, a significant degree of healthcare infrastructure is required ranging from primary care to public health. An example of the multiple facets of a successful vaccine program were outlined in the Mission Indradhanush in India, which planned to make life-saving vaccines available to all children and pregnant women by 2020 through programs with (i) national, (ii) state, (iii) district, and (iv) block/urban level input ( Hinman and McKinlay, 2015 ). National programs require governments to provide financial resources and set out policy for implementation. States needed to obtain the vaccines and to store them appropriately whilst eligible children were identified through public health messaging and outreach. Districts and urban areas recruited staff trained in vaccine delivery and communication to administer vaccines and to provide the aftercare where required. Establishing this degree of nationwide infrastructure to reach those in urban and rural areas, provides the basis for the provision of other health and social care services for all members of the community, in particular improving maternal and infant mortality in developing regions and in the elderly in industrialized regions ( Shearley, 1999 ). Public health infrastructure and personnel could be used to promote other important messages and health education ( Shearley, 1999 ), relating to malnutrition, hygiene and sanitation and preventable diseases such as malaria and HIV infection. Global drivers are also key, as demonstrated by the establishment of the EPI in 1974, when all countries were directed to provide these vaccines, thereby developing their primary- and public health-care infrastructure, with benefit beyond the vaccine program. Vaccination contributes to the UN Millennium Development Goals and later Sustainable Development Goals for achievement by 2030. Gavi, the Vaccine Alliance, has been an important provider of funds, vaccines and support for countries whose gross national income per capita was <£1000/year ( Hinman and McKinlay, 2015 ). The partnerships forged through the development of vaccine programs in LMIC, can be long-lasting and beneficial through other health and social care endeavors ( Shearley, 1999 ).
Impact of Life Expectancy and Opportunity
Vaccination programs provide a degree of social mobility, as poverty and the associated ill-health and mortality from infectious diseases are no longer the determinants of one’s life chances. Vaccine recipients have the potential for improved life-expectancy largely demonstrated by, but not confined to, infants and children ( Andre et al., 2008 ). It has become increasingly recognized that an aging population goes through the process of immunosenescence ( Fulop et al., 2017 ), and increased incidence and severity of infectious diseases. In many countries, therefore, older people are offered vaccines to prevent infections with high mortality and morbidity, including the influenza, pneumococcal, herpes zoster, and pertussis vaccines ( Bonanni et al., 2015 ). These prevent the development of pneumonia, admission to hospital and the subsequent associated risks of death from cardiac failure, as observed in Sweden ( Christenson et al., 2004 ).
The global and interconnected world of the 21st century provides opportunity to discover new cultures, new environments and their resident microbes. The safety of global travel has been greatly enhanced by the availability of vaccines that provide protection against organisms that are different to those in a person’s home setting. Movement of people may be through necessity when fleeing war and conflict, in the search of better life opportunities, or for leisure purposes. For mass movements of refugees vaccines are crucial to the aid and relief efforts to support these individuals ( Hermans et al., 2017 ), as measles and cholera can be highly problematic in refugee camps. Global mass cultural or religious gatherings, such as the Hajj pilgrimage ( Yezli et al., 2018 ) or the Chinese New Year ( Chen et al., 2018 ) have been implicated in the spread of meningococcal disease outbreaks. Pre-travel vaccines offer the optimal level of protection for those with scheduled travel plans and include protection against: yellow fever, hepatitis A and B, rabies, Japanese encephalitis, tick-borne encephalitis, typhoid, and cholera.
Empowerment of Women
The empowerment of women is both a driver and effect of vaccination programs. The degree of education, literacy and independence of girls and women varies considerably across the world and within countries. Where women have the information and autonomy to make health-related decision for their children, childhood immunization rates improve. In a study in Bihar State in rural India involving an empowerment program, where participating women were educated about health and hygiene, there was a higher rate of DTP, measles and BCG vaccination in their children compared to the non-participants in the villages running the program ( Janssens, 2011 ). Further, this information and autonomy served to improve the rates of vaccination in children of non-participants in the villages running the program compared to control villages not running the education program, through social or formal ongoing dialogue within the village community. A separate public health initiative in Haryana, India conducted between 2005 and 2012 to reduce maternal and child health inequalities, involved improving access and provision of health resources to rural areas, the poor in society, women and children. One significant outcome of this initiative was the equitable provision of immunizations to girls and boys, despite the male-favored disparity prior to starting the public health initiative ( Gupta et al., 2016 ).
By improving infant and childhood mortality from infection, more children will survive to adulthood with the potential to have productive and healthy lives. This has led to healthy and economically secure women having fewer children and less peripartum morbidity and mortality ( Sah, 1991 ; Shearley, 1999 ). Thus, women are able to spend more time with their children and on their development ( Shearley, 1999 ) as well as their own education and contribution to the workforce. The strategy of maternal vaccination has demonstrated great success at preventing diseases that afflict infants too young to be vaccinated against pertussis, influenza and tetanus ( Marchant et al., 2017 ). Factors influencing the uptake of maternal vaccination include women’s previous experiences with healthcare and vaccines, so it is crucial to provide the access and support required to enable them to make informed choices during their pregnancy ( Wilson et al., 2019 ).
The impact of vaccines is broad and far-reaching, though not consistently quantifiable, analyzed or communicated. Traditionally, the perceived benefits of vaccination were to reduce morbidity and mortality from infections, and those remain the drivers for the innovation of new vaccines, in particular in preparation for outbreaks or against infections that afflict the most disadvantaged in society. However, an increasing appreciation for the economic and social effects of vaccines is being included in the development and assessment of vaccine programs, potentially realizing a greater benefit to society and resulting in wider implementation. There remain challenges to delivering vaccines to all children and vulnerable people worldwide, in particular those in communities that are difficult to reach geographically, politically and culturally and these challenges can only be overcome with the continued commitment and dedication to this endeavor on an international, national and individual scale.
Author Contributions
SP conceptualized and designed the study. CR prepared the manuscript and figures. CR and SP contributed to literature search and revision and review of the final manuscript. Both authors contributed to the article and approved the submitted version.
Conflict of Interest
SP consults for many major vaccine manufacturers and biotechnology companies but this article was unfunded.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aaby, P., Bhuiya, A., Nahar, L., Knudsen, K., de Francisco, A., and Strong, M. (2003). The survival benefit of measles immunization may not be explained entirely by the prevention of measles disease: a community study from rural Bangladesh. Intern. J. Epidemiol. 32, 106–116.
Google Scholar
Abadom, T. R., Smith, A. D., Tempia, S., Madhi, S. A., Cohen, C., and Cohen, A. L. (2016). Risk factors associated with hospitalisation for influenza-associated severe acute respiratory illness in South Africa: a case-population study. Vaccine 34, 5649–5655.
Anderson, P., Peter, G., Johnston, R. B. Jr., Wetterlow, L. H., and Smith, D. H. (1972). Immunization of humans with polyribophosphate, the capsular antigen of Hemophilus influenzae , type b. J. Clin. Invest. 51, 39–44.
Andre, F. E., Booy, R., Bock, H. L., Clemens, J., Datta, S. K., John, T. J., et al. (2008). Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ. 86, 140–146.
Artenstein, M. S. (1975). Control of meningococcal meningitis with meningococcal vaccines. Yale J. Biol. Med. 48, 197–200.
Austrian, R. (1989). Pneumococcal polysaccharide vaccines. Rev. Infect. Dis. 11, (Suppl. 3), S598–S602.
Barham, T., and Calimeria, L. (2008). Long-term Effects of Family Planning and Child Health Interventions on Adolescent Cognition: Evidence from Matlab in Bangladesh. Boulder, CO: Univeristy of Colorado.
Barnighausen, T., Bloom, D. E., Cafiero-Fonseca, E. T., and O’Brien, J. C. (2014). Valuing vaccination. Proc. Nat. Acad. Sci. U.S.A. 111, 12313–12319.
Bärnighausen, T., Bloom, D. E., Canning, D., Friedman, A., Levine, O. S., O’Brien, J., et al. (2011). Rethinking the benefits and costs of childhood vaccination: the example of the Haemophilus influenzae type b vaccine. Vaccine 29, 2371–2380.
Barrett, S. (2004). Eradication versus control: the economics of global infectious disease policies. Bull. World Health Organ. 82, 683–688.
Bishai, D., Koenig, M., and Ali Khan, M. (2003). Measles vaccination improves the equity of health outcomes: evidence from Bangladesh. Health Econ. 12, 415–419.
Bloom, D. E., and Canning, D. (2000). Policy forum: public health. The health and wealth of nations. Science 287:1207.
Bloom, D. E., Canning, D., and Seiguer, E. (2011). The Effect of Vaccination on Children’s Physical and Cognitive Development in the Philippines. Boston, MA: Harvard School of Public Health.
Bloom, D. E., Canning, D., and Weston, M. (2005). The value of vaccination. World Econ. 6, 15–16.
Bogler, Y., Wong, R. J., and Gish, R. G. (2018). “Epidemiology and natural history of chronic hepatitis B virus infection,” in Hepatitis B Virus and Liver Disease , eds J.-H. Kao and D.-S. Chen (Berlin: Springer), 63–89.
Bonanni, P., Picazo, J. J., and Remy, V. (2015). The intangible benefits of vaccination - what is the true economic value of vaccination? J. Mark. Access Health Policy 3:10.3402/jmahv3.26964.
Borgia, G., Carleo, M. A., Gaeta, G. B., and Gentile, I. (2012). Hepatitis B in pregnancy. World J. Gastroenterol. 18, 4677–4683.
Brinkac, L., Voorhies, A., Gomez, A., and Nelson, K. E. (2017). The threat of antimicrobial resistance on the human microbiome. Microb. Ecol. 74, 1001–1008.
Calmette, A. (1927). La Vaccination Preìventive Contre La Tuberculose par le “BCG,”. Paris: Masson.
Centers for Disease Control and Prevention (2017). Reported Cases Of Notifiable Diseases And Rates Per 100,000, Excluding U.S. Territories, United States, 2017. Atlanta: Centers for Disease Control and Prevention.
Chan, J. F., Yuan, S., Kok, K. H., To, K. K., Chu, H., Yang, J., et al. (2020). A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395, 514–523.
Chen, M., Rodrigues, C. M. C., Harrison, O. B., Zhang, C., Tan, T., Chen, J., et al. (2018). Invasive meningococcal disease in Shanghai, China from 1950 to 2016: implications for serogroup B vaccine implementation. Sci. Rep. 8:12334.
Christenson, B., Hedlund, J., Lundbergh, P., and Ortqvist, A. (2004). Additive preventive effect of influenza and pneumococcal vaccines in elderly persons. Eur. Resp. J. 23, 363–368.
Clark, H. F., Offit, P. A., Plotkin, S. A., and Heaton, P. M. (2006). The new pentavalent rotavirus vaccine composed of bovine (strain WC3) -human rotavirus reassortants. Pediatr. Infect. Dis. J. 25, 577–583.
Curns, A. T., Steiner, C. A., Barrett, M., Hunter, K., Wilson, E., and Parashar, U. D. (2010). Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J. Infect. Dis. 201, 1617–1624.
Daugla, D. M., Gami, J. P., Gamougam, K., Naibei, N., Mbainadji, L., Narbe, M., et al. (2014). Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study [corrected]. Lancet 383, 40–47.
Deogaonkar, R., Hutubessy, R., van der Putten, I., Evers, S., and Jit, M. (2012). Systematic review of studies evaluating the broader economic impact of vaccination in low and middle income countries. BMC Public Health 12:878. doi: 10.1186/s12916-017-0911-878
CrossRef Full Text | Google Scholar
Dominguez, A., Castilla, J., Godoy, P., Delgado-Rodriguez, M., Saez, M., Soldevila, N., et al. (2013). Benefit of conjugate pneumococcal vaccination in preventing influenza hospitalization in children: a case-control study. Pediatr. Infect. Dis. J. 32, 330–334.
Donaghy, M., Cameron, J. C., and Friederichs, V. (2006). Increasing incidence of mumps in Scotland: options for reducing transmission. J. Clin. Virol. 35, 121–129.
Ehreth, J. (2003). The global value of vaccination. Vaccine 21, 596–600.
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., et al. (2012). Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN. Int. J. Cancer 136, E359–E386.
Fine, P. E., and Griffiths, U. K. (2007). Global poliomyelitis eradication: status and implications. Lancet 369, 1321–1322.
Fulop, T., Larbi, A., Dupuis, G., Le Page, A., Frost, E. H., Cohen, A. A., et al. (2017). Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 8:1960. doi: 10.3389/fmicb.2018.01960
PubMed Abstract | CrossRef Full Text | Google Scholar
Funk, S., Knapp, J. K., Lebo, E., Reef, S. E., Dabbagh, A. J., Kretsinger, K., et al. (2019). Combining serological and contact data to derive target immunity levels for achieving and maintaining measles elimination. BMC Med. 17:180. doi: 10.1186/s12889-019-6655-180
Geddes, A. M. (2006). The history of smallpox. Clin. Dermatol. 24, 152–157.
Gentile, I., and Borgia, G. (2014). Vertical transmission of hepatitis B virus: challenges and solutions. Intern. J. Women Health 6, 605–611.
Gessner, B. D., Kaslow, D., Louis, J., Neuzil, K., O’Brien, K. L., Picot, V., et al. (2017). Estimating the full public health value of vaccination. Vaccine 35, 6255–6263.
GHRF Commission (2016). Commission on a Global Health Risk Framework for the Future). The Neglected Dimension of Global Security A Framework to Counter Infectious Disease Crises. Commission on Global Health Risk Framework for the Future. Available online at: https://nam.edu/wp-content/uploads/2016/01/Neglected-Dimension-of-Global-Security.pdf (September 24, 2019).
Glenny, A. T., and Hopkins, B. E. (1923). Diphtheria toxoid as an immunising agent. Br. J. Exp. Pathol. 4, 283–288.
Global Polio Eradication Initiative (2019). Polio this Week, Wild Poliovirus Type 1 And Circulating Vaccine-Derived Poliovirus Cases. Available online at: http://polioeradication.org/polio-today/polio-now/this-week/ (accessed June 25, 2020).
Gold, R., and Artenstein, M. S. (1971). Meningococcal infections. 2. Field trial of group C meningococcal polysaccharide vaccine in 1969-70. Bull. World Health Organ. 45, 279–282.
Gouglas, D., Thanh, Le, T., Henderson, K., Kaloudis, A., Danielsen, T., et al. (2018). Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. Lancet Glob. Health 6:e001386-96.
Grizas, A. P., Camenga, D., and Vazquez, M. (2012). Cocooning: a concept to protect young children from infectious diseases. Curr. Opin. Pediatr. 24, 92–97.
Gupta, G., Glueck, R., and Patel, P. R. (2017). HPV vaccines: global perspectives. Hum. Vacc. Immunotherap. 13, 1–4.
Gupta, M., Angeli, F., Bosma, H., Rana, M., Prinja, S., Kumar, R., et al. (2016). Effectiveness of multiple-strategy community intervention in reducing geographical, socioeconomic and gender based inequalities in maternal and child health outcomes in Haryana, India. PLoS One 11:e0150537. doi: 10.1371/journal.pone.0150537
Hajjeh, R. (2011). Accelerating introduction of new vaccines: barriers to introduction and lessons learned from the recent Haemophilus influenzae type B vaccine experience. Philos. Trans. R. Soc. B. 366, 2827–2832.
Hermans, M. P. J., Kooistra, J., Cannegieter, S. C., Rosendaal, F. R., Mook-Kanamori, D. O., and Nemeth, B. (2017). Healthcare and disease burden among refugees in long-stay refugee camps at Lesbos. Greece. Eur. J. Epidemiol. 32, 851–854.
Heukelbach, J., Alencar, C. H., Kelvin, A. A., de Oliveira, W. K., and Pamplona de Goes, C. L. (2016). Zika virus outbreak in Brazil. J. Infect. Dev. Countr. 10, 116–120.
Hill, H. A., Elam-Evans, L. D., Yankey, D., Singleton, J. A., and Kang, Y. (2017). Vaccination coverage among children aged 19-35 Months - United States, 2016. MMWR Morb. Mort. Wkly Rep. 66, 1171–1177.
Hinman, A. R., and McKinlay, M. A. (2015). Immunization equity. Vaccine 33, (Suppl. 4), D72–D77.
Hotez, P. J. (2001). Vaccines as instruments of foreign policy. The new vaccines for tropical infectious diseases may have unanticipated uses beyond fighting diseases. EMBO Rep. 2, 862–868.
Janssens, W. (2011). Externalities in program evaluation: the impact of a Women’s empowerment program on immunization. J. Eur. Econ. Assoc. 9, 1082–1113.
Jenner, E. (1798). An Inquiry Into The Causes And Effects Of The Variolæ Vaccinæ, A Disease Discovered In Some Of The Western Counties Of England, Particularly Gloucestershire, And Known By The Name Of The Cow Pox. London: Sampson Low.
Jit, M., Hutubessy, R., Png, M. E., Sundaram, N., Audimulam, J., Salim, S., et al. (2015). The broader economic impact of vaccination: reviewing and appraising the strength of evidence. BMC Med. 13:209. doi: 10.1186/s12916-017-0911-209
Keja, K., Chan, C., Hayden, G., and Henderson, R. H. (1988). Expanded programme on immunization. World Health Stat. Q. 41, 59–63.
Kirnbauer, R., Booy, F., Cheng, N., Lowy, D. R., and Schiller, J. T. (1992). Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Nat. Acad. Sci. U.S.A. 89, 12180–12184.
Kyaw, M. H., Lynfield, R., Schaffner, W., Craig, A. S., Hadler, J., Reingold, A., et al. (2006). Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae . New Engl. J. Med. 354, 1455–1463.
Levine, M. M., and Robins-Browne, R. (2009). Vaccines, global health and social equity. Immunol. Cell Biol. 87, 274–278.
Lien, G., and Heymann, D. L. (2013). The problems with polio: toward eradication. Infect. Dis. Ther. 2, 167–174.
Lieu, T. A., Cochi, S. L., Black, S. B., Halloran, M. E., Shinefield, H. R., Holmes, S. J., et al. (1994). Cost-effectiveness of a routine varicella vaccination program for US children. JAMA 271, 375–381.
Liu, Y. Y., Wang, Y., Walsh, T. R., Yi, L. X., Zhang, R., Spencer, J., et al. (2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16, 161–168.
Logan, L. K., and Weinstein, R. A. (2017). The epidemiology of carbapenem-resistant Enterobacteriaceae : the impact and evolution of a global menace. J. Infect. Dis. 215(Suppl._1), S28–S36.
Maassab, H. F., and DeBorde, D. C. (1985). Development and characterization of cold-adapted viruses for use as live virus vaccines. Vaccine 3, 355–369.
Madhi, S. A., Klugman, K. P., and Vaccine Trialist, G. (2004). A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat. Med. 10, 811–813.
Maiden, M. C., Ibarz-Pavon, A. B., Urwin, R., Gray, S. J., Andrews, N. J., Clarke, S. C., et al. (2008). Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J. Infect. Dis. 197, 737–743.
Manzoli, L., Schioppa, F., Boccia, A., and Villari, P. (2007). The efficacy of influenza vaccine for healthy children: a meta-analysis evaluating potential sources of variation in efficacy estimates including study quality. Pediatr. Infect. Dis. J. 26, 97–106.
Marchant, A., Sadarangani, M., Garand, M., Dauby, N., Verhasselt, V., Pereira, L., et al. (2017). Maternal immunisation: collaborating with mother nature. Lancet. Infect. Dis. 17, e197–e208. doi: 10.1016/S1473-3099(17)30229-3
Mina, M. J., Kula, T., Leng, Y., Li, M., de Vries, R. D., Knip, M., et al. (2019). Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science 366, 599–606.
Mina, M. J., Metcalf, C. J., de Swart, R. L., Osterhaus, A. D., and Grenfell, B. T. (2015). Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science 348, 694–699.
Morens, D. M., and Fauci, A. S. (2007). The 1918 influenza pandemic: insights for the 21st century. J. Infect. Dis. 195, 1018–1028.
Morens, D. M., Holmes, E. C., Davis, A. S., and Taubenberger, J. K. (2011). Global rinderpest eradication: lessons learned and why humans should celebrate too. J. Infect. Dis. 204, 502–505.
Moss, W. J. (2017). Measles. Lancet 390, 2490–2502.
Munira, S. L., and Fritzen, S. A. (2007). What influences government adoption of vaccines in developing countries? A policy process analysis. Soc. Sci. Med. 65, 1751–1764.
Needham, J. (2000). Science and Civilisation in China: Volume 6, Biology and Biological Technology. Cambridge: Cambridge University Press.
Niessen, L. W., ten Hove, A., Hilderink, H., Weber, M., Mulholland, K., and Ezzati, M. (2009). Comparative impact assessment of child pneumonia interventions. Bull. World Health Organ. 87, 472–480.
Nunes, M. C., Cutland, C. L., Jones, S., Downs, S., Weinberg, A., Ortiz, J. R., et al. (2017). Efficacy of maternal influenza vaccination against all-cause lower respiratory tract infection hospitalizations in young infants: results from a randomized controlled trial. Clin. Infect. Dis. 65, 1066–1071.
Orenstein, W. A., and Ahmed, R. (2017). Simply put: vaccination saves lives. Proc. Nat. Acad. Sci. U.S.A. 114, 4031–4033.
Ozawa, S., Clark, S., Portnoy, A., Grewal, S., Brenzel, L., and Walker, D. G. (2016). Return On investment from childhood immunization in low- and middle-income countries, 2011-20. Health Aff. 35, 199–207.
Palmer, T., Wallace, L., Pollock, K. G., Cuschieri, K., Robertson, C., Kavanagh, K., et al. (2019). Prevalence of cervical disease at age 20 after immunisation with bivalent HPV vaccine at age 12-13 in Scotland: retrospective population study. BMJ 365:l1161.
Palmu, A. A., Jokinen, J., Nieminen, H., Rinta-Kokko, H., Ruokokoski, E., Puumalainen, T., et al. (2014). Effect of pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) on outpatient antimicrobial purchases: a double-blind, cluster randomised phase 3-4 trial. Lancet Infect. Dis. 14, 205–212.
Pasteur, L. (1880). De l’attenuation du virus du choléra des poules. Comptes Rendus De l’Acad. Sci. 91, 673–680.
Pasteur, L. (1881). Sur la vaccination charbonneuse. Comptes Rendus De l’Acad. Sci. 92, 1378–1383.
Pasteur, L. (1885). Mèthode pour prévenir la rage apres morsure. Comptes Rendus De l’Acad. Sci. 101, 765–772.
Paulke-Korinek, M., Kundi, M., Rendi-Wagner, P., de Martin, A., Eder, G., Schmidle-Loss, B., et al. (2011). Herd immunity after two years of the universal mass vaccination program against rotavirus gastroenteritis in Austria. Vaccine 29, 2791–2796.
Peltola, H., Aavitsland, P., Hansen, K. G., Jonsdottir, K. E., Nokleby, H., and Romanus, V. (1999). Perspective: a five-country analysis of the impact of four different Haemophilus influenzae type b conjugates and vaccination strategies in Scandinavia. J. Infect. Dis. 179, 223–229.
Petrova, V. N., Sawatsky, B., Han, A. X., Laksono, B. M., Walz, L., Parker, E., et al. (2019). Incomplete genetic reconstitution of B cell pools contributes to prolonged immunosuppression after measles. Sci. Immunol. 4:eaay6125.
Plans-Rubio, P. (2012). Evaluation of the establishment of herd immunity in the population by means of serological surveys and vaccination coverage. Hum. Vacc. Immunotherap. 8, 184–188.
Plotkin, S. (2014). History of vaccination. Proc. Natl. Acad. Sci. U.S.A. 111, 12283–12287.
Plotkin, S. A. (2017). Vaccines for epidemic infections and the role of CEPI. Hum. Vacc. Immunotherap. 13, 2755–2762.
Plotkin, S. A., and Mortimer, E. A. (1988). Vaccines. Philadelphia, PA: Saunders.
Pollard, A. J., Perrett, K. P., and Beverley, P. C. (2009). Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat. Rev. Immunol. 9, 213–220.
Poovorawan, Y., Chongsrisawat, V., Theamboonlers, A., Leroux-Roels, G., Kuriyakose, S., Leyssen, M., et al. (2011). Evidence of protection against clinical and chronic hepatitis B infection 20 years after infant vaccination in a high endemicity region. J. Viral Hepat. 18, 369–375.
Public Health England (2018). Public Health Matters Ten Years On Since The Start Of The HPV Vaccine Programme – What Impact Is It Having? London: Public Health England.
Public Health England (2019). Human Papillomavirus (HPV) Vaccination Coverage In Adolescent Females In England: 2017/18. London: Public Health England.
Regan, A. K., de Klerk, N., Moore, H. C., Omer, S. B., Shellam, G., and Effler, P. V. (2016). Effect of maternal influenza vaccination on hospitalization for respiratory infections in newborns: a retrospective cohort study. Pediatr. Infect. Dis. J. 35, 1097–1103.
Riumallo-Herl, C., Chang, A. Y., Clark, S., Constenla, D., Clark, A., Brenzel, L., et al. (2018). Poverty reduction and equity benefits of introducing or scaling up measles, rotavirus and pneumococcal vaccines in low-income and middle-income countries: a modelling study. BMJ Glob. Health 3:e000613.
Roeder, P., Mariner, J., and Kock, R. (2013). Rinderpest: the veterinary perspective on eradication. Philos. Trans. R. Soc. B 368:20120139.
Roush, S. W., and Murphy, T. V. (2007). Vaccine-preventable disease table working G. historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA 298, 2155–2163.
Russo, F. B., Jungmann, P., and Beltrao-Braga, P. C. B. (2017). Zika infection and the development of neurological defects. Cell. Microbiol. 19: 12744.
Sah, R. K. (1991). The effects of child-mortality changes on fertility choice and parental welfare. J. Polit. Econ. 99, 582–606.
Sato, Y., and Sato, H. (1999). Development of acellular pertussis vaccines. Biologicals 27, 61–69.
Schneerson, R., Barrera, O., Sutton, A., and Robbins, J. B. (1980). Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J. Exp. Med. 152, 361–376.
Serruto, D., Bottomley, M. J., Ram, S., Giuliani, M. M., and Rappuoli, R. (2012). The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine 30, (Suppl. 2), B87–B97.
Shearley, A. E. (1999). The societal value of vaccination in developing countries. Vaccine 17, (Suppl. 3), S109–S112.
Simonsen, L., Taylor, R. J., Young-Xu, Y., Haber, M., May, L., and Klugman, K. P. (2011). Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. mBio 2:e00309-10.
Soergel, P., Makowski, L., Schippert, C., Staboulidou, I., Hille, U., and Hillemanns, P. (2012). The cost efficiency of HPV vaccines is significantly underestimated due to omission of conisation-associated prematurity with neonatal mortality and morbidity. Hum. Vacc. Immunother. 8, 243–251.
Strassburg, M. A. (1982). The global eradication of smallpox. Am. J. Infect. Control 10, 53–59.
Tabrizi, S. N., Brotherton, J. M., Kaldor, J. M., Skinner, S. R., Liu, B., Bateson, D., et al. (2014). Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect.ous Dis. 14, 958–966.
Thacker, N., Vashishtha, V. M., and Thacker, D. (2016). Polio eradication in India: the lessons learned. Pediatrics 138:e20160461.
Thanh Le, T., Andreadakis, Z., Kumar, A., Gomez Roman, R., Tollefsen, S., Saville, M., et al. (2020). The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 19, 305–306.
Theiler, M., and Smith, H. H. (1937a). The effect of prolonged cultivation in vitro upon the pathogenicity of yellow fever virus. J. Exp. Med. 65, 767–786.
Theiler, M., and Smith, H. H. (1937b). The use of yellow fever virus modified by in vitro cultivation for human immunization. J. Exp. Med. 65, 787–800.
Turner, H. C., Thwaites, G. E., and Clapham, H. E. (2018). Vaccine-preventable diseases in lower-middle-income countries. Lancet Infect. Dis. 18, 937–939.
United Nations Department of Economic and Social Affairs (2019). Population Division. World Population Prospects 2019. New York, NY: United Nations Department of Economic and Social Affairs.
Van der Wielen, M., Giaquinto, C., Gothefors, L., Huelsse, C., Huet, F., Littmann, M., et al. (2010). Impact of community-acquired paediatric rotavirus gastroenteritis on family life: data from the REVEAL study. BMC Fam. Pract. 11:22. doi: 10.1186/s12916-017-0911-22
Verguet, S., Murphy, S., Anderson, B., Johansson, K. A., Glass, R., and Rheingans, R. (2013). Public finance of rotavirus vaccination in India and Ethiopia: an extended cost-effectiveness analysis. Vaccine 31, 4902–4910.
Wang, X., Huang, X., and Zhang, Y. (2018). Involvement of human papillomaviruses in cervical cancer. Front. Microbiol. 9:2896. doi: 10.3389/fmicb.2018.02896
Whitney, C. G., Farley, M. M., Hadler, J., Harrison, L. H., Bennett, N. M., Lynfield, R., et al. (2003). Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. New Engl. J. Med. 348, 1737–1746.
Wilder-Smith, A., Longini, I., Zuber, P. L., Barnighausen, T., Edmunds, W. J., Dean, N., et al. (2017). The public health value of vaccines beyond efficacy: methods, measures and outcomes. BMC Med. 15:138. doi: 10.1186/s12916-017-0911-8
Wilson, R., Paterson, P., and Larson, H. J. (2019). Strategies to improve maternal vaccination acceptance. BMC Public Health 19:342. doi: 10.1186/s12889-019-6655-y
Wise, J. (2018). Child vaccination rates drop in England as MMR uptake falls for fourth year. BMJ 362:k3967.
Wise, J. (2019). MMR vaccine: johnson urges new impetus to increase uptake as UK loses measles-free status. BMJ 366:l5219.
World Bank (2019). Immunization, DPT (% of Children Ages 12-23 Months). Washington, DC: World Bank.
World Health Assembly (1974). The expanded programme on immunization: the 1974 resolution by the world health assembly. Assign. Child. 1985, 87–88.
World Health Organisation (2019a). Immunization Coverage. Geneva: World Health Organisation.
World Health Organisation (2019b). Polio Eradication. Geneva: World Health Organisation.
World Health Organisation (2019c). Reported Cases of Selected Vaccine Preventable Diseases (VPDs). Geneva: World Health Organisation.
World Health Organization [WHO], United Nations Children’s Fund [UNICEF] (2019). WHO/UNICEF Estimates of National Immunization Coverage (WUENIC) 1980-2018. Geneva: WHO.
Yezli, S., Gautret, P., Assiri, A. M., Gessner, B. D., and Alotaibi, B. (2018). Prevention of meningococcal disease at mass gatherings: lessons from the Hajj and Umrah. Vaccine 36, 4603–4609.
Zhou, F., Shefer, A., Wenger, J., Messonnier, M., Wang, L. Y., Lopez, A., et al. (2009). Economic evaluation of the routine childhood immunization program in the United States. Pediatrics 133, 577–585.
Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., et al. (2020). A novel coronavirus from patients with pneumonia in China, 2019. New Engl. J. Med. 382, 727–733.
Keywords : immunization, vaccines, infectious diseases, infection, children, health economics
Citation: Rodrigues CMC and Plotkin SA (2020) Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 11:1526. doi: 10.3389/fmicb.2020.01526
Received: 09 April 2020; Accepted: 12 June 2020; Published: 14 July 2020.
Reviewed by:
Copyright © 2020 Rodrigues and Plotkin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stanley A. Plotkin, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Jump to navigation
- Bahasa Malaysia

What are the benefits and risks of vaccines for preventing COVID-19?
Key messages
– Most vaccines reduce, or probably reduce, the number of people who get COVID-19 disease and severe COVID-19 disease.
– Many vaccines likely increase number of people experiencing events such as fever or headache compared to placebo (sham vaccine that contains no medicine but looks identical to the vaccine being tested). This is expected because these events are mainly due to the body's response to the vaccine; they are usually mild and short-term.
– Many vaccines have little or no difference in the incidence of serious adverse events compared to placebo.
– There is insufficient evidence to determine whether there was a difference between the vaccine and placebo in terms of death because the numbers of deaths were low in the trials.
– Most trials assessed vaccine efficacy over a short time, and did not evaluate efficacy to the COVID variants of concern.
What is SARS-CoV-2 and COVID-19?
SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) is the virus that causes COVID-19 disease. Not everyone infected with SARS-CoV-2 will develop symptoms of COVID-19. Symptoms can be mild (e.g. fever and headaches) to life-threatening (e.g. difficulty breathing), or death.
How do vaccines prevent COVID-19?
While vaccines work slightly differently, they all prepare the body's immune system to prevent people from getting infected with SARS-CoV-2 or, if they do get infected, to prevent severe disease.
What did we want to find out?
We wanted to find out how well each vaccine works in reducing SARS-CoV-2 infection, COVID-19 disease with symptoms, severe COVID-19 disease, and total number of deaths (including any death, not only those related to COVID-19).
We wanted to find out about serious adverse events that might require hospitalization, be life-threatening, or both; systemic reactogenicity events (immediate short-term reactions to vaccines mainly due to immunological responses; e.g. fever, headache, body aches, fatigue); and any adverse events (which include non-serious adverse events).
What did we do?
We searched for studies that examined any COVID-19 vaccine compared to placebo, no vaccine, or another COVID-19 vaccine.
We selected only randomized trials (a study design that provides the most robust evidence because they evaluate interventions under ideal conditions among participants assigned by chance to one of two or more groups). We compared and summarized the results of the studies, and rated our confidence in the evidence based on factors such as how the study was conducted.
What did we find?
We found 41 worldwide studies involving 433,838 people assessing 12 different vaccines. Thirty-five studies included only healthy people who had never had COVID-19. Thirty-six studies included only adults, two only adolescents, two children and adolescents, and one included adolescents and adults. Three studied people with weakened immune systems, and none studied pregnant women.
Most cases assessed results less than six months after the primary vaccination. Most received co-funding from academic institutions and pharmaceutical companies. Most studies compared a COVID-19 vaccine with placebo. Five evaluated the addition of a 'mix and match' booster dose.
Main results
We report below results for three main outcomes and for 10 World Health Organization (WHO)-approved vaccines (for the remaining outcomes and vaccines, see main text). There is insufficient evidence regarding deaths between vaccines and placebo (mainly because the number of deaths was low), except for the Janssen vaccine, which probably reduces the risk of all-cause deaths.
People with symptoms
The Pfizer, Moderna, AstraZeneca, Sinopharm-Beijing, and Bharat vaccines produce a large reduction in the number of people with symptomatic COVID-19.
The Janssen vaccine reduces the number of people with symptomatic COVID-19.
The Novavax vaccine probably has a large reduction in the number of people with symptomatic COVID-19.
There is insufficient evidence to determine whether CoronaVac vaccine affects the number of people with symptomatic COVID-19 because results differed between the two studies (one involved only healthcare workers with a higher risk of exposure).
Severe disease
The Pfizer, Moderna, Janssen, and Bharat vaccines produce a large reduction in the number of people with severe disease.
There is insufficient evidence about CoronaVac vaccine on severe disease because results differed between the two studies (one involved only healthcare workers with a higher risk of exposure).
Serious adverse events
For the Pfizer, CoronaVac, Sinopharm-Beijing, and Novavax vaccines, there is insufficient evidence to determine whether there was a difference between the vaccine and placebo mainly because the number of serious adverse events was low.
Moderna, AstraZeneca, Janssen, and Bharat vaccines probably result in no or little difference in the number of serious adverse events.
What are the limitations of the evidence?
Most studies assessed the vaccine for a short time after injection, and it is unclear if and how vaccine protection wanes over time. Due to the exclusion criteria of COVID-19 vaccine trials, results cannot be generalized to pregnant women, people with a history of SARS-CoV-2 infection, or people with weakened immune systems. More research is needed comparing vaccines and vaccine schedules, and effectiveness and safety in specific populations and outcomes (e.g. preventing long COVID-19). Further, most studies were conducted before the emergence of variants of concerns.
How up to date is this evidence?
The evidence is up to date to November 2021. This is a living systematic review. Our results are available and updated bi-weekly on the COVID-NMA platform at covid-nma.com.
Compared to placebo, most vaccines reduce, or likely reduce, the proportion of participants with confirmed symptomatic COVID-19, and for some, there is high-certainty evidence that they reduce severe or critical disease. There is probably little or no difference between most vaccines and placebo for serious adverse events. Over 300 registered RCTs are evaluating the efficacy of COVID-19 vaccines, and this review is updated regularly on the COVID-NMA platform ( covid-nma.com ).
Implications for practice
Due to the trial exclusions, these results cannot be generalized to pregnant women, individuals with a history of SARS-CoV-2 infection, or immunocompromized people. Most trials had a short follow-up and were conducted before the emergence of variants of concern.
Implications for research
Future research should evaluate the long-term effect of vaccines, compare different vaccines and vaccine schedules, assess vaccine efficacy and safety in specific populations, and include outcomes such as preventing long COVID-19. Ongoing evaluation of vaccine efficacy and effectiveness against emerging variants of concern is also vital.
Different forms of vaccines have been developed to prevent the SARS-CoV-2 virus and subsequent COVID-19 disease. Several are in widespread use globally.
To assess the efficacy and safety of COVID-19 vaccines (as a full primary vaccination series or a booster dose) against SARS-CoV-2.
We searched the Cochrane COVID-19 Study Register and the COVID-19 L·OVE platform (last search date 5 November 2021). We also searched the WHO International Clinical Trials Registry Platform, regulatory agency websites, and Retraction Watch.
We included randomized controlled trials (RCTs) comparing COVID-19 vaccines to placebo, no vaccine, other active vaccines, or other vaccine schedules.
We used standard Cochrane methods. We used GRADE to assess the certainty of evidence for all except immunogenicity outcomes.
We synthesized data for each vaccine separately and presented summary effect estimates with 95% confidence intervals (CIs).
We included and analyzed 41 RCTs assessing 12 different vaccines, including homologous and heterologous vaccine schedules and the effect of booster doses. Thirty-two RCTs were multicentre and five were multinational. The sample sizes of RCTs were 60 to 44,325 participants. Participants were aged: 18 years or older in 36 RCTs; 12 years or older in one RCT; 12 to 17 years in two RCTs; and three to 17 years in two RCTs. Twenty-nine RCTs provided results for individuals aged over 60 years, and three RCTs included immunocompromized patients. No trials included pregnant women. Sixteen RCTs had two-month follow-up or less, 20 RCTs had two to six months, and five RCTs had greater than six to 12 months or less. Eighteen reports were based on preplanned interim analyses.
Overall risk of bias was low for all outcomes in eight RCTs, while 33 had concerns for at least one outcome.
We identified 343 registered RCTs with results not yet available.
This abstract reports results for the critical outcomes of confirmed symptomatic COVID-19, severe and critical COVID-19, and serious adverse events only for the 10 WHO-approved vaccines. For remaining outcomes and vaccines, see main text. The evidence for mortality was generally sparse and of low or very low certainty for all WHO-approved vaccines, except AD26.COV2.S (Janssen), which probably reduces the risk of all-cause mortality (risk ratio (RR) 0.25, 95% CI 0.09 to 0.67; 1 RCT, 43,783 participants; high-certainty evidence).
Confirmed symptomatic COVID-19
High-certainty evidence found that BNT162b2 (BioNtech/Fosun Pharma/Pfizer), mRNA-1273 (ModernaTx), ChAdOx1 (Oxford/AstraZeneca), Ad26.COV2.S, BBIBP-CorV (Sinopharm-Beijing), and BBV152 (Bharat Biotect) reduce the incidence of symptomatic COVID-19 compared to placebo (vaccine efficacy (VE): BNT162b2: 97.84%, 95% CI 44.25% to 99.92%; 2 RCTs, 44,077 participants; mRNA-1273: 93.20%, 95% CI 91.06% to 94.83%; 2 RCTs, 31,632 participants; ChAdOx1: 70.23%, 95% CI 62.10% to 76.62%; 2 RCTs, 43,390 participants; Ad26.COV2.S: 66.90%, 95% CI 59.10% to 73.40%; 1 RCT, 39,058 participants; BBIBP-CorV: 78.10%, 95% CI 64.80% to 86.30%; 1 RCT, 25,463 participants; BBV152: 77.80%, 95% CI 65.20% to 86.40%; 1 RCT, 16,973 participants).
Moderate-certainty evidence found that NVX-CoV2373 (Novavax) probably reduces the incidence of symptomatic COVID-19 compared to placebo (VE 82.91%, 95% CI 50.49% to 94.10%; 3 RCTs, 42,175 participants).
There is low-certainty evidence for CoronaVac (Sinovac) for this outcome (VE 69.81%, 95% CI 12.27% to 89.61%; 2 RCTs, 19,852 participants).
Severe or critical COVID-19
High-certainty evidence found that BNT162b2, mRNA-1273, Ad26.COV2.S, and BBV152 result in a large reduction in incidence of severe or critical disease due to COVID-19 compared to placebo (VE: BNT162b2: 95.70%, 95% CI 73.90% to 99.90%; 1 RCT, 46,077 participants; mRNA-1273: 98.20%, 95% CI 92.80% to 99.60%; 1 RCT, 28,451 participants; AD26.COV2.S: 76.30%, 95% CI 57.90% to 87.50%; 1 RCT, 39,058 participants; BBV152: 93.40%, 95% CI 57.10% to 99.80%; 1 RCT, 16,976 participants).
Moderate-certainty evidence found that NVX-CoV2373 probably reduces the incidence of severe or critical COVID-19 (VE 100.00%, 95% CI 86.99% to 100.00%; 1 RCT, 25,452 participants).
Two trials reported high efficacy of CoronaVac for severe or critical disease with wide CIs, but these results could not be pooled.
Serious adverse events (SAEs)
mRNA-1273, ChAdOx1 (Oxford-AstraZeneca)/SII-ChAdOx1 (Serum Institute of India), Ad26.COV2.S, and BBV152 probably result in little or no difference in SAEs compared to placebo (RR: mRNA-1273: 0.92, 95% CI 0.78 to 1.08; 2 RCTs, 34,072 participants; ChAdOx1/SII-ChAdOx1: 0.88, 95% CI 0.72 to 1.07; 7 RCTs, 58,182 participants; Ad26.COV2.S: 0.92, 95% CI 0.69 to 1.22; 1 RCT, 43,783 participants); BBV152: 0.65, 95% CI 0.43 to 0.97; 1 RCT, 25,928 participants). In each of these, the likely absolute difference in effects was fewer than 5/1000 participants.
Evidence for SAEs is uncertain for BNT162b2, CoronaVac, BBIBP-CorV, and NVX-CoV2373 compared to placebo (RR: BNT162b2: 1.30, 95% CI 0.55 to 3.07; 2 RCTs, 46,107 participants; CoronaVac: 0.97, 95% CI 0.62 to 1.51; 4 RCTs, 23,139 participants; BBIBP-CorV: 0.76, 95% CI 0.54 to 1.06; 1 RCT, 26,924 participants; NVX-CoV2373: 0.92, 95% CI 0.74 to 1.14; 4 RCTs, 38,802 participants).
For the evaluation of heterologous schedules, booster doses, and efficacy against variants of concern, see main text of review.
Should COVID-19 vaccines be mandatory? Two experts discuss
Senior Research Fellow, Oxford Uehiro Centre for Practical Ethics, University of Oxford
NIHR Academic Clinical Fellow in Public Health Medicine, UCL
Disclosure statement
Alberto Giubilini receives funding from the Arts and Humanities Research Council/UK Research and Innovation (AHRC/UKRI) and has previously received funding from the Wellcome Trust.
Vageesh Jain is affiliated with Public Health England under an honorary contract as a speciality registrar.
University College London provides funding as a founding partner of The Conversation UK.
University of Oxford provides funding as a member of The Conversation UK.
View all partners

To be properly protective, COVID-19 vaccines need to be given to most people worldwide. Only through widespread vaccination will we reach herd immunity – where enough people are immune to stop the disease from spreading freely. To achieve this, some have suggested vaccines should be made compulsory , though the UK government has ruled this out . But with high rates of COVID-19 vaccine hesitancy in the UK and elsewhere , is this the right call? Here, two experts to make the case for and against mandatory COVID-19 vaccines.
Alberto Giubilini, Senior Research Fellow, Oxford Uehiro Centre for Practical Ethics, University of Oxford
COVID-19 vaccination should be mandatory – at least for certain groups. This means there would be penalties for failure to vaccinate, such as fines or limitations on freedom of movement.
The less burdensome it is for an individual to do something that prevents harm to others, and the greater the harm prevented, the stronger the ethical reason for mandating it.
Being vaccinated dramatically reduces the risk of seriously harming or killing others. Vaccines such as the Pfizer , AstraZeneca or Moderna ones with 90-95% efficacy at preventing people from getting sick are also likely to be effective at stopping the virus from spreading, though possibly to a lower degree. Such benefits would come at a very minimal cost to individuals.
Lockdown is mandatory. Exactly like mandatory vaccination, it protects vulnerable people from COVID-19. But, as I have argued in detail elsewhere, unlike mandatory vaccination, lockdown entails very large individual and societal costs. It is inconsistent to accept mandatory lockdown but reject mandatory vaccination. The latter can achieve a much greater good at a much smaller cost.
Also, mandatory vaccination ensures that the risks and burdens of reaching herd immunity are distributed evenly across the population. Because herd immunity benefits society collectively, it’s only fair that the responsibility of reaching it is shared evenly among society’s individual members.
Of course, we might achieve herd immunity through less restrictive alternatives than making vaccination mandatory – such as information campaigns to encourage people to be vaccinated. But even if we reach herd immunity, the higher the uptake of vaccines, the lower the risk of falling below the herd immunity threshold at a later time. We should do everything we can to prevent that emergency from happening – especially when the cost of doing so is low.
Fostering trust and driving uptake by making people more informed is a nice narrative, but it’s risky. Merely giving people information on vaccines does not always result in increased willingness to vaccinate and might actually lower confidence in vaccines. On the other hand, we’ve seen mandatory vaccination policies in Italy recently successfully boost vaccine uptake for other diseases.
Mandatory seatbelt policies have proven very successful in reducing deaths from car accidents, and are now widely endorsed despite the (very small) risks that seatbelts entail. We should see vaccines as seatbelts against COVID-19. In fact, as very special seatbelts, which protect ourselves and protect others.

Vageesh Jain, NIHR Academic Clinical Fellow in Public Health Medicine, UCL
Mandatory vaccination does not automatically increase vaccine uptake. An EU-funded project on epidemics and pandemics, which took place several years before COVID-19, found no evidence to support this notion. Looking at Baltic and Scandinavian countries, the project’s report noted that countries “where a vaccination is mandatory do not usually reach better coverage than neighbour or similar countries where there is no legal obligation”.
According to the Nuffield Council of Bioethics, mandatory vaccination may be justified for highly contagious and serious diseases. But although contagious, Public Health England does not classify COVID-19 as a high-consequence infectious disease due to its relatively low case fatality rate.
COVID-19 severity is strongly linked with age, dividing individual perceptions of vulnerability within populations. The death rate is estimated at 7.8% in people aged over 80, but at just 0.0016% in children aged nine and under. In a liberal democracy, forcing the vaccination of millions of young and healthy citizens who perceive themselves to be at an acceptably low risk from COVID-19 will be ethically disputed and is politically risky.
Public apprehensions for a novel vaccine produced at breakneck speed are wholly legitimate. A UK survey of 70,000 people found 49% were “very likely” to get a COVID-19 vaccine once available. US surveys are similar . This is not because the majority are anti-vaxxers.
Despite promising headlines, the trials and pharmaceutical processes surrounding them have not yet been scrutinised. With the first trials only beginning in April , there is limited data on long-term safety and efficacy. We don’t know how long immunity lasts for. None of the trials were designed to tell us if the vaccine prevents serious disease or virus transmission.
To disregard these ubiquitous concerns would be counterproductive. As a tool for combating anti-vaxxers – estimated at around 58 million globally and making up a small minority of those not getting vaccinated – mandatory vaccines are also problematic. The forces driving scientific and political populism are the same . Anti-vaxxers do not trust experts, industry and especially not the government. A government mandate will not just be met with unshakeable defiance, but will also be weaponised to recruit others to the anti-vaxxer cause.
In the early 1990s, polio was endemic in India , with between 500 and 1,000 children getting paralysed daily. By 2011, the virus was eliminated. This was not achieved through legislation. It was down to a consolidated effort to involve communities, target high-need groups, understand concerns, inform, educate, remove barriers, invest in local delivery systems and link with political and religious leaders.
Mandatory vaccination is rarely justified. The successful roll-out of novel COVID-19 vaccines will require time, communication and trust. We have come too far, too fast, to lose our nerve now.
- Mandatory vaccination
- Coronavirus
- Vaccine hesitancy
- Coronavirus insights

Lecturer / Senior Lecturer in Indigenous Knowledges

Investigator, Student Academic Misconduct (Multiple Positions Available)

Commissioning Editor Nigeria

Professor in Physiotherapy

Postdoctoral Research Associate

IMAGES
VIDEO
COMMENTS
Conclusion; Essay Title 3: "The Impact of Vaccine Disinformation on Public Health: A Global Challenge" Thesis Statement: The proliferation of vaccine disinformation poses a significant threat to public health, and addressing this challenge is vital to ensure widespread vaccine acceptance and disease control. Essay Outline: Introduction
Vaccines are especially important for at-risk populations such as young children and older adults. The AAFP offers vaccination recommendations, immunization schedules, and information on disease-specific vaccines. Being up to date on vaccines is especially important as children head back to school.
Vaccines are among the most safe and effective public health interventions to prevent serious disease and death. Because of the success of vaccines, most Americans today have no firsthand experience with such devastating illnesses as polio or diphtheria.
Create an outline: Organize your essay with an introduction, body paragraphs, and a conclusion. Explain the topic: Use facts and examples to explain the chosen aspect of COVID-19 in detail. Maintain objectivity: Present information in a neutral and unbiased manner.
Conclusion. The impact of vaccines is broad and far-reaching, though not consistently quantifiable, analyzed or communicated.
The COVID-19 pandemic has led the world to undertake the largest vaccination campaign in human history. In record time, unprecedented scientific and governmental efforts have resulted in the acquisition of immunizers utilizing different technologies ...
Conclusion. Restatement of thesis: Research shows that the benefits of vaccination outweigh the risks because vaccines can prevent serious illness and disease in individuals, vaccinations can also prevent widespread outbreaks of diseases in populations and the side effect of vaccinations, though occasionally serious, are very rare.
Future research should evaluate the long-term effect of vaccines, compare different vaccines and vaccine schedules, assess vaccine efficacy and safety in specific populations, and include outcomes such as preventing long COVID-19.
Conclusions. Most of the COVID-19 vaccines appear to be effective and safe. Double-dose vaccination is recommended. However, more research is needed to investigate the long-term efficacy and safety of the vaccines and the influence of dose, age, and production process on the protective efficacy.
COVID-19 vaccination should be mandatory – at least for certain groups. This means there would be penalties for failure to vaccinate, such as fines or limitations on freedom of movement.